Tenofovir Alafenamide versus Tenofovir Disoproxil Fumarate: Systematic Review and Meta-Analysis-Juniper publishers
JUNIPER PUBLISHERS-OPEN
ACCESS INTERNATIONAL JOURNAL OF PULMONARY & RESPIRATORY SCIENCES
Abstract
Background: Highly active antiretroviral
therapy (HAART) has greatly reduced morbidity and mortality. Despite the
impact of antiretroviral therapy (ART), mortality in successfully
treated HIV infected patients remains higher than in the general
uninfected population, more specifically in Sub-Saharan Africa. In fact,
ART has demonstrated toxicity. tenofovir disoproxil (TDF) is widely
known, even so, TDF is known as nephrotoxic. Recently, tenofovir
alafenamide (TAF) was found. TAF is a new oral prodrug of tenofovir,
less toxic than TDF. TAF has potential intracellular accumulation; lower
extracellular exposures of tenofovir may be realized with the potential
to reduce off-target toxicities. Additionally, TAF has shown its
efficacy in HIV-Hepatitis B co-infection.
Objectives: To investigate whether TAF based
regimens are less renal and borne toxic than TDF based regimens. To
evaluate the efficacy of TAF versus TDF in HIV/hepatitis B co-infection.
Methods: We searched in studies in following
databases: CENTRAL (Cochrane Central Register of Controlled Trials),
Scopus, Web of science, LILACS, PubMed and CINAHL. We also searched
conference abstract through HIV/AIDS website.
Main results: Among 916 studies found in
different databases, 764 were screened after removing duplicate studies,
36 studies were included in qualitative studies. Among them, 16 studies
were excluded with specific reasons. 18 RCTs were included in
meta-analysis. Of the 12 RTCs assessing the OR of HIV-RNA<50RNAc/ml
from 48 to 96 weeks, HIV-infected patients on TAF based regimens reduced
HIV-RNA<50RNAc/ ml by 13% compared to TDF contained group (P=0.02).
For 10 RCTs included in clearance creatinine rate comparing TAF to TFD
based regimens, the glomerular filtration rate yielded a pooled MD
estimate of -3.94(-6.07 to-1.81, P<0.000001). The OR of HBV- DNA
after 48 weeks between TAF and TDF was reduced by 29% (4 RCTs were
included) with P=0.02. TDF individuals had a low MD of CD4 count
(cells/μl) than TAF group (MD -18.99, 6 studies, P<00001). The MD of
percentage change hip bone mineral density was decreased in TFD compared
to TAF -1.93 with P<0.00001 and 11 RCTs were included as well as the
MD of percentage change spine bone mineral density was decreased in TFD
compared to TAF -1.77 (-1.97 to -1.58) with P=0.001. The odds of ALT
above ULN was reduced by 25% in TAF group compared to TDF group
(P=0.04). Any adverse events and serious adverse events were not
significant in both TAF and TDF groups. We graded the evidence as high
in all outcomes except in bone Mineral Density and proteinuria where the
evidence was respectively low and moderate.
Conclusion: Evidence suggests that use of TAF
is more protective and effective than either TDF. Improving renal and
hepatic related comorbidities in HIV-infected population, TAF may be
beneficial in public health policy, specifically in high HIV epidemic
regions.
Keywords: Tenofovir alafenamide; Tenofovir disoproxil fumarate; HIV; Hepatitis B
Abbreviations: HAART:
Highly Active Antiretroviral Therapy; ART: Antiretroviral Therapy; TDF:
Antiretroviral Therapytenofovir Disoproxil; TAF: Tenofovir Alafenamide;
HAART: Highly Active Antiretroviral Therapy; PrEP: Pre-Exposure
Prophylaxis; PI: Protease Inhibitors; GFR: Filtration Rate; Cr:
Creatinine; GFR: Glomerular Filtration Rate; AKI: Acute Kidney Injury;
CKDs: Chronic Kidney Diseases; GFR: Glomerular Filtration Rate
Introduction
HIV epidemic still carries a huge burden of morbidity
and mortality in a wide part of the world, and according to the
estimates of the Joint United Nations Programme on HIV/AIDS [1], 36.7 million [30.8 million-42.9 million] people worldwide were living with HIV in 2016 [1]. In the same year [1.6 million-2.1 million] people were newly infected with HIV. Among them,
830 000 to 1.2 million died from acquired immunodeficiency syndrome (AIDS)-related causes [1]. 20.9 million People were accessing antiretroviral therapy in June 2017 [1].
The vast majority of people living with HIV are located in low- and
middle- income countries, with an estimated 25.5 million living in
sub-Saharan Africa. Among this group 19.4 million are living in East and
Southern Africa which saw 44% of new HIV infections globally in 2016 [2].
The region most affected by HIV epidemic is still
sub-Saharan Africa where 4.9% of the adult population is HIV-infected,
and the region itself accounts for 69% of people living with HIV
globally [2].
The revolution of Highly active antiretroviral therapy (HAART) has
greatly reduced morbidity and mortality, resulting then in high survival
rates among infected patients [3-5].
Despite the impact of HAART, mortality and morbidity in successfully
treated HIV infected patients remains higher than HIV uninfected
population [2].
In fact, the effects of persistent inflammation in HIV infected
population, antiretroviral toxicity on comorbidities that are related to
HIV infection, including metabolic, cardiovascular and renal disease,
contribute actively in high mortality and morbidity among HIV infected
population [3-5].
Reviewing the literature and clinical data in Sub Saharan Africa, drug
toxicity related mortality and mortality is occupying an important
grade. Among those antiretroviral drugs, tenofovir disoproxil fumarate
(TDF) is widely used. TDF-containing combinations antiretroviral therapy
(ART) is currently considered as the first-line regimens for HIV
treatment and prevention of mother-to-child transmission (PMTCT) Option
B/B+ [6]. Moreover, TDF is the approved drug for pre-exposure prophylaxis (PrEP) [6-9].
Knowing TDF nephrotoxicity, regular clearance creatinine monitoring is
crucial in ART initiation and schedule monitoring. However, most
Sub-Saharan African countries lack regular monitoring of clearance
creatinine. Consequently, chronic kidney diseases prevalence are topping
up in this region.
Recently, a systematic review has demonstrated that
TDF- containing regimens were associated with a significantly greater
loss of kidney function. Furthermore, the review also found a
significantly higher risk of acute renal injury associated with TDF use [9].
In spite of that, debate continues over whether more widespread use of
TDF, particularly in "real world" clinical settings, might yet reveal a
risk for nephrotoxicity significant enough to limit its use or to
necessitate close clinical monitoring [7-9].
Previous studies have reported several risk factors for TDF-induced
nephrotoxicity. Among them, include high basal serum creatinine (Cr)
level, simultaneous use of nephrotoxic drugs, low body weight, old age,
hypertension, diabetes mellitus and low CD4+ T cell count [10,11].
It is presumed that proximal tubule damage, decreased bone density, and
reduced glomerular filtration rate (GFR) could also occur in
association with TDF use [12,13]. Besides, the combinations with protease inhibitors (PI), specifically atazanavir can an additional decrease in GFR.
As a matter of fact, HIV-related renal diseases are one of the leading causes of chronic kidney diseases (CKDs) worldwide [14].
CKD is defined by a sustained change in urinary sediment, such as the
presence of proteinuria, or by a reduced glomerular filtration rate
(GFR) [15].
Nephrotoxicity can appear either during long or short-term use of TDF.
TDF-induced nephrotoxicity is reported in about 15% of patients treated
with TDF for 2-9 years
[16.17] . TDF can also cause acute kidney injury (AKI), proximal tubular
dysfunction, or both in combination [18].
In addition, interstitial nephritis, renal tubular damage, and
nephrolithiasis have been detected as renal complications of HIV
infection
[16.17] . Proteinuria is often the earliest manifestation of CKD and is
more common in HIV-infected individuals than in similarly aged
HIV-negative controls [18].
Recently, Tenofovir alafenamide (TAF), a new oral prodrug of tenofovir,
a nucleotide analogue that inhibits HIV-1 transcription was found [19].
This prodrug is already used in America, Europe and Oceania.
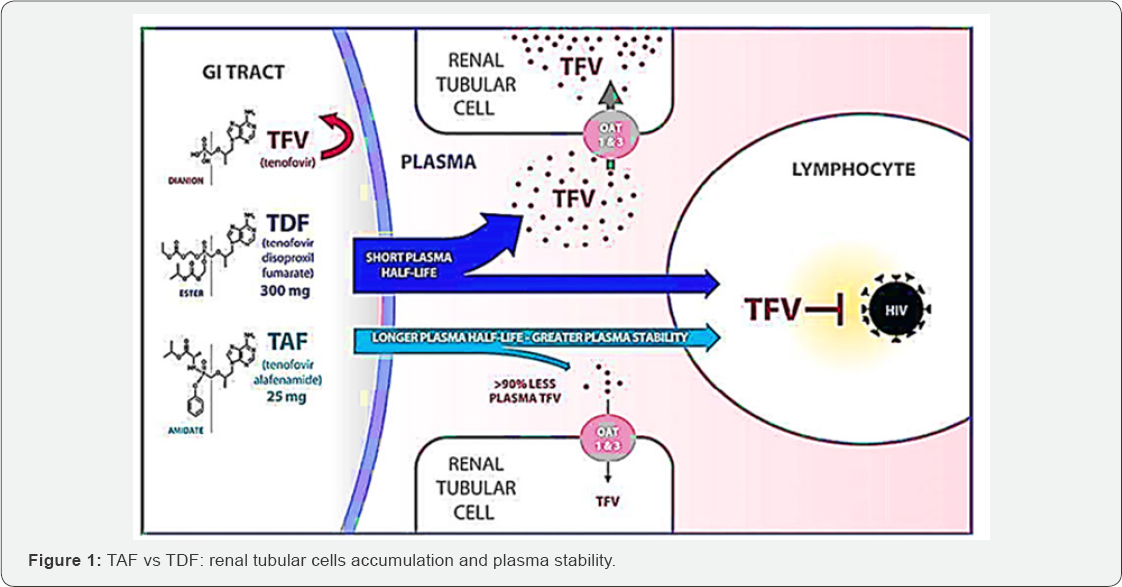
Experimental studies have illustrated that TAF is more stable in plasma
than TDF (Figure 1)
and then is specifically converted into tenofovir within cells by the
cellular enzyme cathepsin A, which is highly expressed in lymphoid
tissues (Figure 1) [20].
Tenofovir is then further metabolized intracellularly to the active
metabolite, tenofovir diphosphate, a competitive inhibitor of HIV-1
reverse transcriptase that terminates the elongation of the nascent
viral cDNA chain [21].
Given the intracellular mechanism of activation of TAF and potential
for intracellular accumulation, by the way, lower extracellular
exposures may be realized with the potential to reduce renal toxicities [21].
Specifically, lower drug exposures to kidney cells may provide for
fewer renal complications as observed in a minority of patients treated
with TDF and the ability to dose TAF in patients with renal impairment
without dose adjustment [9,16-19].
That is why, TAF was identified as an alternate TFV prodrug to TDF that more efficiently loads HIV-target cells [21]. A recent study demonstrated that TAF is 1000- and 10-fold more active against HIV in vitro than TFV or TDF, respectively [21].
The majority of intact TAF transits directly into its lymphoid cell
target, where it is then converted intracellularly to tenofovir
diphosphate [22-24].
Following dosing with TAF, the resulting systemic exposure to TFV is
91% lower than is the case for an equipotent dose of TDF [25,26]. This in-target cell conversion of prodrug minimizes systemic exposure to TFV [27].
TAF is not a substrate for renal organic anion transporters and this,
along with the lower plasma levels of TFV, has been demonstrated to
confer a better renal safety profile than that associated with TDF [27].
TAF was recently approved for the treatment of HIV-1 infection in the US and EU as part of the single-tablet regimen [19].
The evidence to date suggests that this TAF-containing regimen offers
high virological success rates that are similar to those of TDF- based
regimens, with a more favorable safety and tolerability profile,
characterized by less impact on multiple measures of renal function and
less impact on bone mineral density (BMD) in both treatment-naive and
treatment experienced patients [28].
Indeed, data from studies in virologically suppressed patients with
either normal renal function or mild to moderate renal impairment (eGFR
30-69mL/min), suggest that TAF may offer TFV-equivalent potency together
with an improved renal and bone safety profile.
Besides, this review emphasize the role of TAF in
HIV/ hepatitis B co-infection. In fact, chronic hepatitis B virus (HBV)
infection is one of the leading causes of cirrhosis, liver
decompensating, and hepatocellular carcinoma (HCC). An estimated 257
million people are positive for hepatitis B surface antigen (HBsAg)
globally. HIV-Hepatitis B co-infection is common and TDF based regimens
are the most used to control chronic hepatitis B. Both TAF and TDF are
phosphonoamidate prodrugs of tenofovir (TFV) that share the same
intracellular active metabolite, TFV diphosphate (TFV-DP), which is
effective against both HBV and HIV-1 infection [29-31].
However, TAF has greater plasma stability as shown above, allowing then
more efficient TAF uptake by hepatocytes at lower plasma concentrations
than TDF (Figure 1),
thus the circulating concentration of TFV is 90% lower after
administration of a 25 mg dose of TAF than after a 300 mg dose of TDF [32-34].
Studies have shown that the efficacy of TAF was not inferior to that of
TDF for both HBeAg-positive and -negative patients in regards to
virologic outcomes [35,36].
However, the rate of (alanine transaminase) ALT normalization by the
more stringent American Association Study of Liver Diseases (AASLD)
criteria was significantly higher for TAF than for TDF. This systematic
review is crucial in its genre because the results could play a role of
turnover in changing the use of TDF to TAF, decreasing then
nephrotoxicity due to TDF based regimens in both HIV-infected and not
infected with HIV in the case post exposure prophylaxis [39].
In addition, other fields are investigated among which HIV viral load,
CD4 count and bone mineral density. Moreover, this study is also focused
on comparing TAF to TDF to control HIV-hepatitis B co-infection.
Objectives
a. To evaluate the efficacy of TAF based regimens are compared to TDF based regimens.
b. To investigate whether TAF based regimens are less renal and borne toxic than TDF based regimens.
c. To compare whether TAF contained regimens is more effective in HIV/HIB co-infection compared to TDF.
This systematic review was reported in accordance
with the Preferred Reporting Items for Systematic Review and Meta-
Analyses statement. A protocol was registered with international
prospective register of systematic reviews (PROSPERO) (identification
number: CRD42016032717). This protocol could be found online at
http://www.crd.york.ac.uk/PROSPERO/
display_record.asp?ID=CRD42016032717.
Included studies definitions
For all included studies, the intervention was TAF-
contained regimens and the control group was TDF contained regimens. We
included only parallel randomized control trials in which the
participants were HIV-infected adult patients. The outcomes were
included viral load, serum clearance creatinine, proteinuria, HBV DNA
and HBsAg as primary outcomes and secondary outcomes were bone mineral
density, CD4 count, hepatic transminases and adverse events.
Search strategy, selection criteria, data extraction
CENTRAL (Cochrane Central Register of Controlled
Trials), Scopus, Web of science, LILACS, PubMed, CINAHL and MEDLINE were
systematically searched without language, publication or date
restrictions using key words and MeSH designed MeSH descriptor HIV
Infections explode all trees AND MeSH descriptor HIV explode all trees
AND (hiv OR hiv-1* OR hiv-2* OR hiv1 OR hiv2 OR hiv infect* OR human
imminodeficiency virus OR human immune-deficiency virus OR human
immuno-deficiency virus OR human immun* deficiency virus OR acquired
immunodeficiency syndrome) AND (tenofovir OR TNF OR TDF OR PMPA OR
Tenofovir Disoproxil OR Tenofovir Disoproxil Fumarate OR (Disoproxil
Fumarate, Tenofovir) OR Fumarate, Tenofovir Disoproxil OR Viread) AND
(Tenofovir alafenamide OR tenofovir prodrug OR TAF OR Vimlidy) AND
(Randomized controlled trial) OR (controlled clinical trial) OR
(randomized controlled trials) OR (random allocation) OR (double-blind
method) OR (single-blind method) OR (clinical trial) OR (trial) OR
(clinical trials) OR (clinical trial) OR (singl* OR doubl*) OR (trebl*
OR tripl*) AND (mask* OR blind*) OR (placebos) OR (placebo*) OR
(random*) on June December 2017. A combination of MeSH and ENTREE
headings were used with free-text terms to enhance the sensitivity of
the search. We further search conference abstract archives on the web
sites of the Conference on Retroviruses and Opportunistic Infections
(CROI), the International AIDS Conference (IAC), and the International
AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention
(IAS). All references in review articles found by our database search
were included using Revman Software [40].
Three investigators (JLT, LMM and JLT) independently screened and
assessed titles and abstracts for inclusion. Full texts were
independently assessed for inclusion and study type by JLT and JLT with
disagreements resolved by discussion. JLT, LMM and JLT extracted the
data. The methodology used for collecting and analyzing data was based
on the guidance of the Cochrane Handbook of Systematic Reviews of
Interventions [41].
JLT and JLT worked independently reviewed the abstracts of all studies
identified through database searches or other resources. Full texts of
the articles were obtained for closer examination.
Data extraction sheets were recorded: first author,
study design publication year, study years, study settings country,
trial identification number, published or unpublished, Follow up:
duration and completeness of follow up, Study power, Details of
participants(Baseline: age range; gender; CD4 count, viral load, HIV
stage, Details of treatment), outcomes(primary and secondary) and Risk
of bias assessment. We solved missing data in different ways. We
obtained the standard deviations (SDs) from standard errors, confidence
intervals, t-value and p-values. However, some studies did not report
the SDs. Then, we contacted study authors to obtain missing data. Three
RCTs included missing data. We deal with these issues by using
amputation [41]. Quality of individual studies was assessed using the Cochrane tool for randomized control trials with six domains [41]: Sequence generation: how the allocation sequence was generated and described whether it was adequate
a. Allocation concealment: how the allocation sequence was concealed and clarified whether it was adequate.
b. Blinding of participants, personnel, and outcome assessors.
c. The description of the completeness of outcome data for each outcome.
d. Selective outcome reporting was assessed and
funnel plots were generated in case that the outcome included ten or
more studies.
e. Other potential sources of bias. Two reviewers (JLT and LMM) assessed independently the risk of bias in included study.
Each domain, the quality was graded and reported as
high, low, or moderate risk of bias. In addition, we assessed reporting
bias by using the funnel plots respectively for HIV-RNA<50RNAc/ ml,
Glomerular filtration rate (ml/min), Mean percentage change Hip Bone
Mineral Density (%) and Mean percentage change Spine Bone Mineral
Density (%).
We found that data from studies are as similar as possible and then we combined in Cochrane's Review Manager Software [40]
for meta-analysis for the different outcomes. The study populations,
interventions, outcomes and study designs were sufficiently similar
across the studies' critical appraisal. This is why we pooled the data
across studies and estimate summary effect sizes using both fixed- and
random effects models. When assessing outcome, for continuous outcomes
(serum clearance creatinine, CD4, Mean percentage change Bone Mineral
Density), we used mean differences and its 95% CI, and for dichotomous
outcomes (HIV-RNA<50, HBV DNA, Virological Failure, Proteinuria, ALT
above ULN, and adverse events), we compared proportions in TAF and TDF
group using the odd ratio and it 95% CI.
The I2 test of heterogeneity was performed to ensure
that the differences between the results of each RCT could not be
expected by chance. Where we find substantial heterogeneity (I2 greater
than 50%), we investigated main reasons for the heterogeneity. By the
way, subgroup analysis was undertaken. Subgroups analysis was performed
by HIV-RNA baseline and different TAF and TDF regimens
(Duranavir/cobicistat/TAF versus Duranavir/cobicistat/TDF;
Elvitegravir/cobicistat/TAF versus Elvitegravir/cobicistat/TDF and
Efavirenz/Elvitegravir/
TAF versus Efavirenz/Elvitegravir/TDF). HIV-RNA<50RNAc/ ml,
Glomerular filtration rate (ml/min) and Mean percentage change Bone
Mineral Density (%) included more than 10 RCTs in meta-analysis, then we
produced funnel plots to assess evidence of publication bias. We
performed Egger test in case that the funnel plots were asymmetric. All
statistical analyses were undertaken using Revman [40,41]
statistical software. However, we handled missing data and publication
bias by using STATA version 14. GRADE evidence profiles and summary of
findings tables was assessed using Grade profile software. We graded
different results as high, moderate, low or very low evidence based on
studies designs included in meta-analysis, risk of bias, inconsistency,
and indirectness and imprecision.
Results
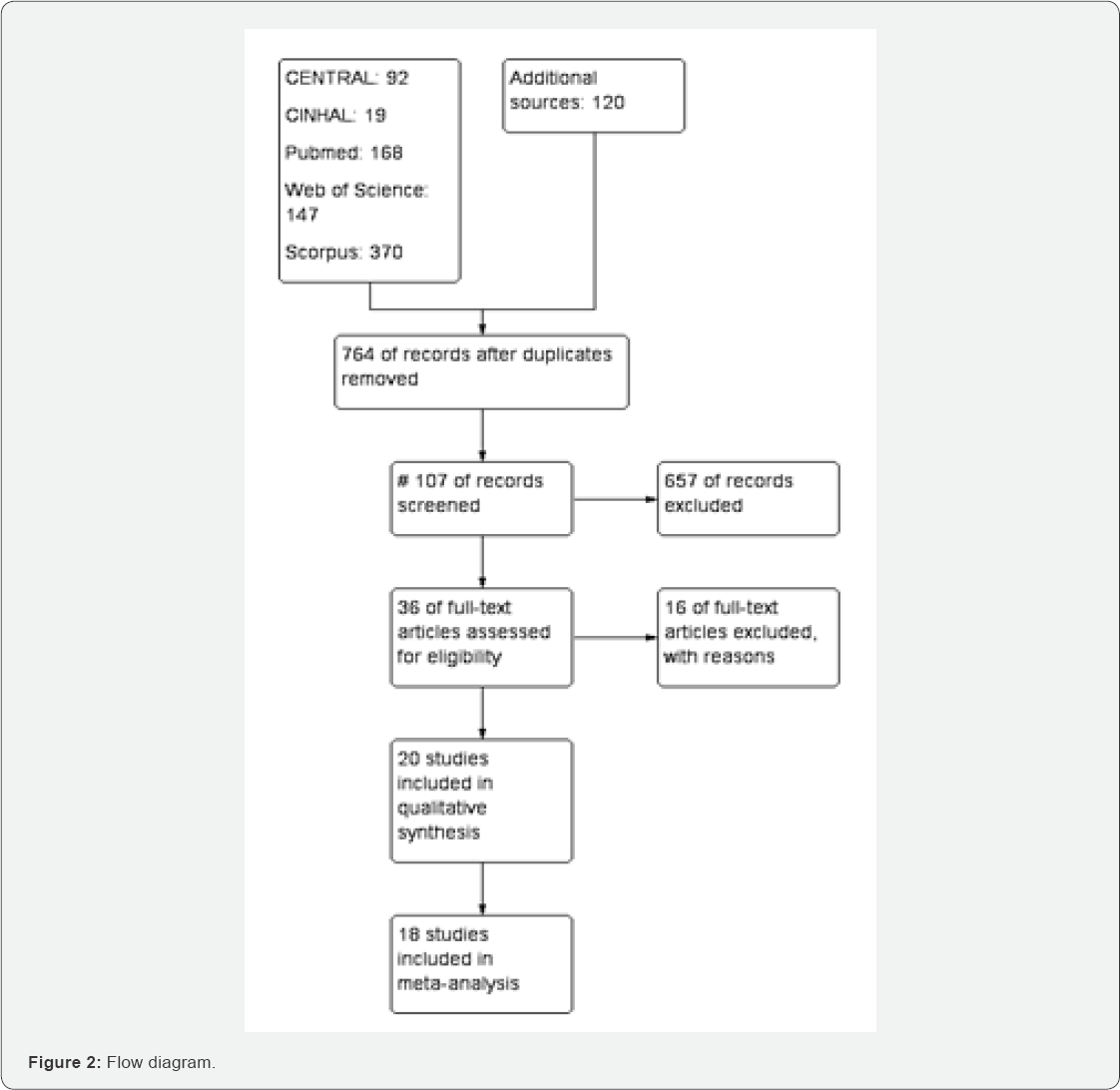
Of the 916 studies found in different database, 764
were screened after removing duplicate studies, 36 studies were included
in qualitative studies (Figure 2). Among them, 16 studies [25,29,30,42-54] were excluded with specific reasons. [38] is an ongoing study. 18 studies [26,31,55-70] were included in meta-analysis (Figure 2). Characteristics of included and excluded studies are described in annexed tables (Figure 2).
Meta-analysis
HIV-RNA<50, Virological Failure, HBV DNA, HBeg,
Glomerular filtration rate (ml/min), Proteinuria, CD4 cells/ ml, Mean
percentage change Hip Bone Mineral Density (%), Mean percentage change
Spine Bone Mineral Density (%), any adverse events, Serious adverse
events and ALT above ULN were assessed through meta-analysis.
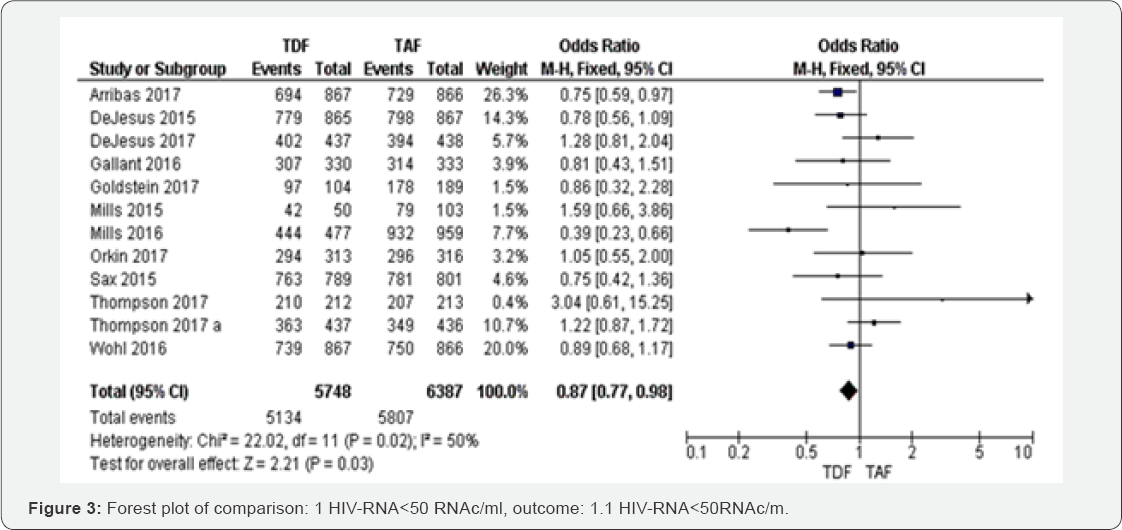
HIV-RNA<50RNAc/ml (48 to 144 weeks): Of the 12
RTCs assessing HIV-RNA<50RNAc/ml from 48 to 96 weeks, the fixed-
effects meta-analysis of HIV-infected patients on TDF based regimens
compared TAF contained regimens gave an OR of 0.87 (95% CI 0.7 to 0.98,
P=0.02) with I2=50% (Figure 3). The overall evidence was graded as high.
Virological failure (48 to 144 weeks): Among the five
studies that included in meta-analysis of virological failure, TAF
group was less likely to treatment failure compared to TDF (OR 0.92, 95%
CI 0.65 to 1.29).
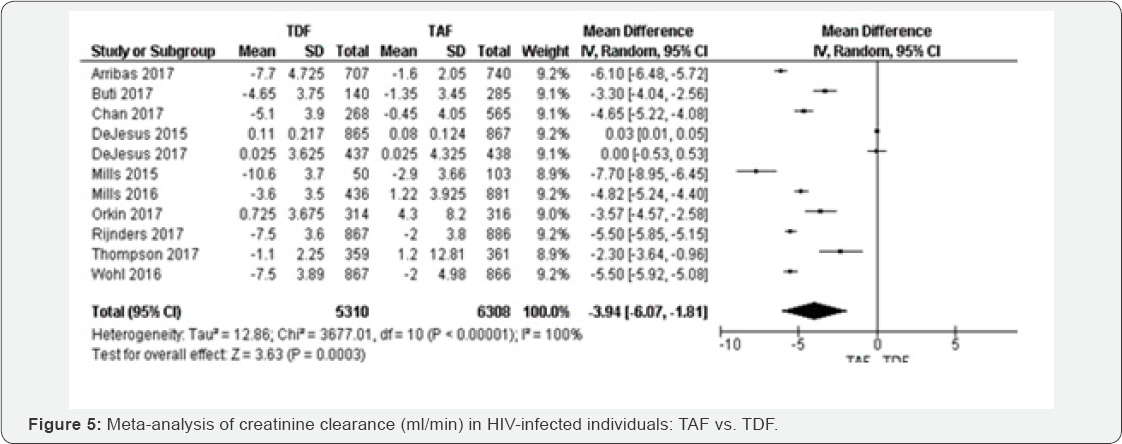
Creatinine Clearance rate(ml/min) (48 to 144 weeks):
For 10 RCTs included in creatinine clearance rate comparing TAF to TFD
based regimens, the random-effects meta-analysis of glomerular
filtration rate yielded a pooled MD estimate of -3.94( 95% CI -6.07
to-1.81, P<0.000001) with I2=100% (Figure 4).
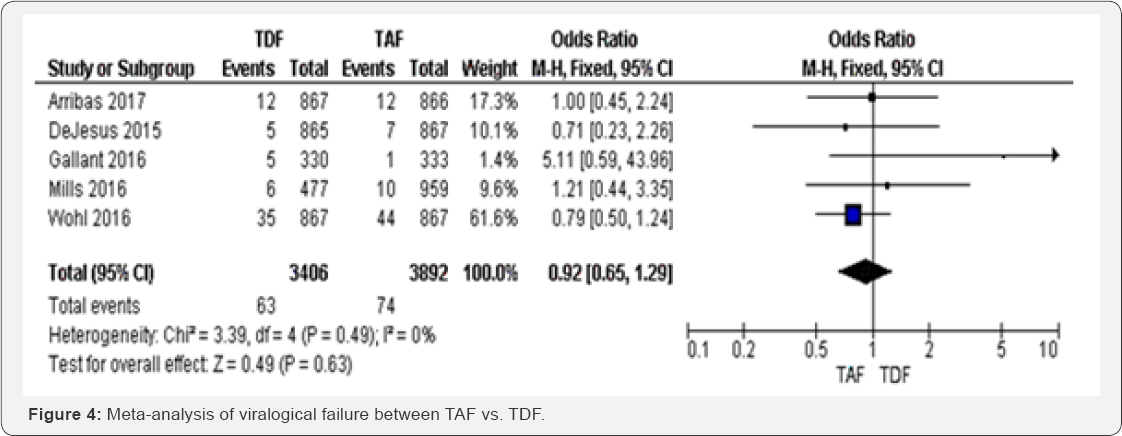
Therefore, the results were not statistically significant with P=0.63. The results were homogenous with I2=0% (Figure 4).
The evidence was judged as high. We graded the evidence as low.
Statistical heterogeneity was high between included studies;
this is was subgroup analysis was undertaken for justification.
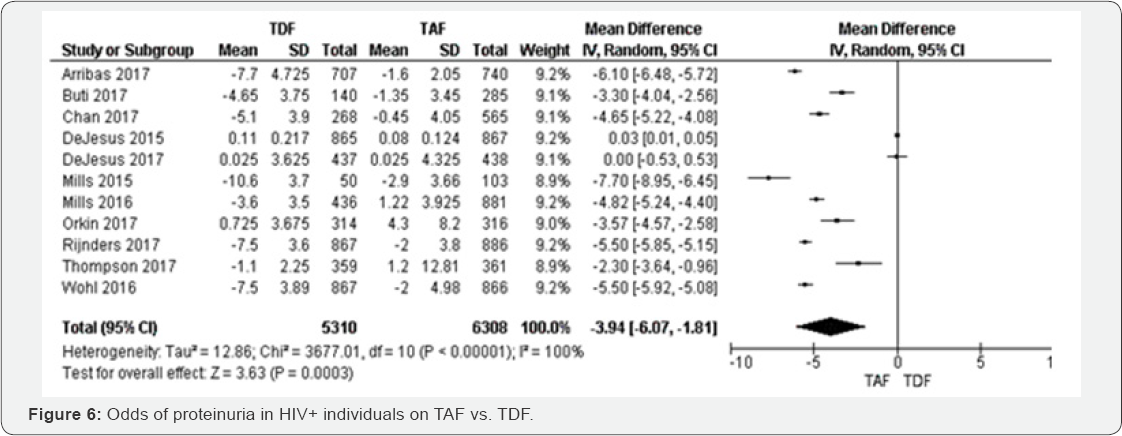
Proteinuria (48 to 144 weeks): Compared to individuals on TAF contained
regimens, proteinuria was higher in TDF group OR 1.11 (95% CI 0.8 1 to
1.54, P=0.03), with high quality of
evidence. The results were heterogeneous (I2=64%), but the five
individual point estimates were independently significant (Figure 5).
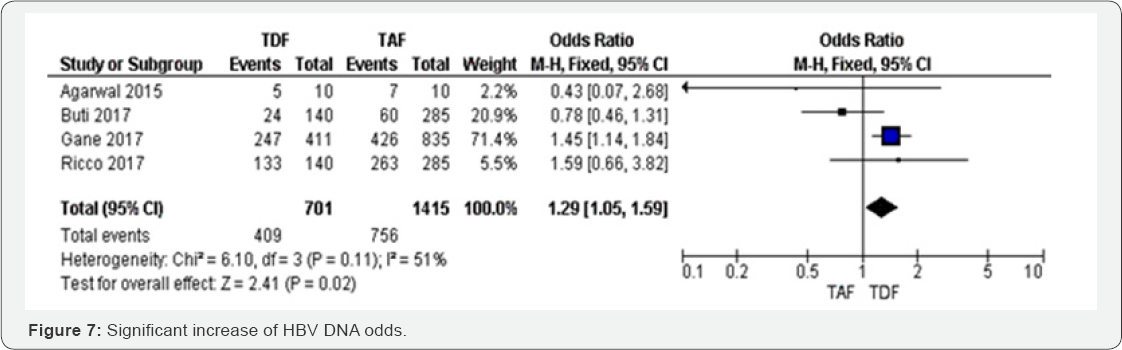
HBV DNA after 96 weeks: Four RCTs were included in
metaanalysis assessing HBV DNA between TAF and TDF. The results were
only significant in one study which weight was high, and then this study
influenced the overall results OR 1.29 (95%CI 1.05 to 1.59, P=0.02).
There were three smallest studies that reported a non-significant
increase of HBV DNA odds. Varying the estimation method from random
effect to fixed model did not change the statistical significance but it
reduced the point estimate by 7%. However, HBV DNA was decreased by 29 %
in TAF group compared to TDF group. The results were moderately
heterogeneous (I2 = 51%), mostly due to the three studies that showed a
point estimate included the null value. The overall evidence was judged
as high (Figure 6 & 7).
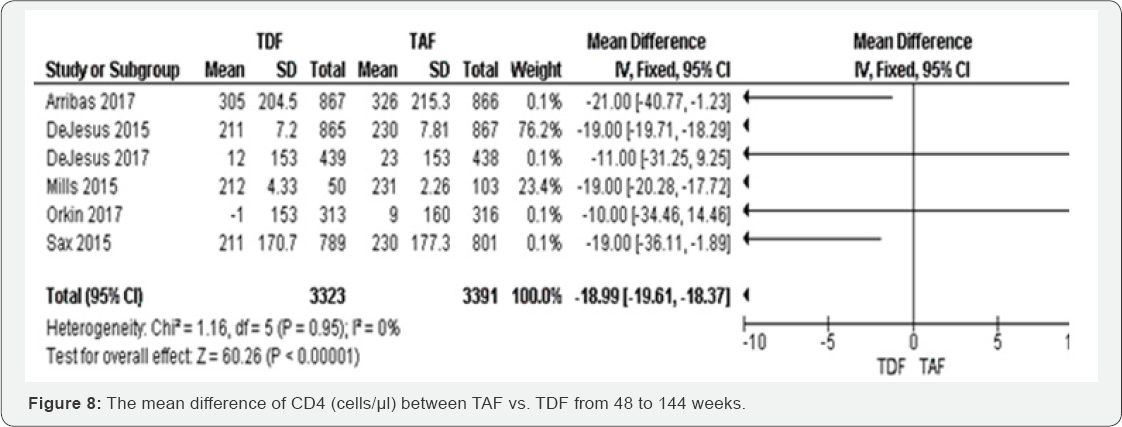
CD4 count (cells/μl) (48 to 144 weeks): TDF
individuals had a low MD of CD4 count than TAF group (MD -18.99, 95% CI
-19.61,18.37, P<00001). Among six included studies, the results were
consistent four studies, with higher point estimates (Arribas 2017;
Dejesus 2015; Mills 2015; Sax 2015). These results were graded as high
evidence. The results were homogenous (I2=0%) and insensitive to the
effect estimation method. The mean difference of percentage change hip
BMD was decreased in TFD compared to TAF -1.93 (-2.21 to -1.65) with
P<0.00001. These results have shown low evidence that hip BMD is more
likely to decrease in TDF group compared to TAF group. The results were
highly heterogeneous (I2=89%) (Figure 8 & 9).
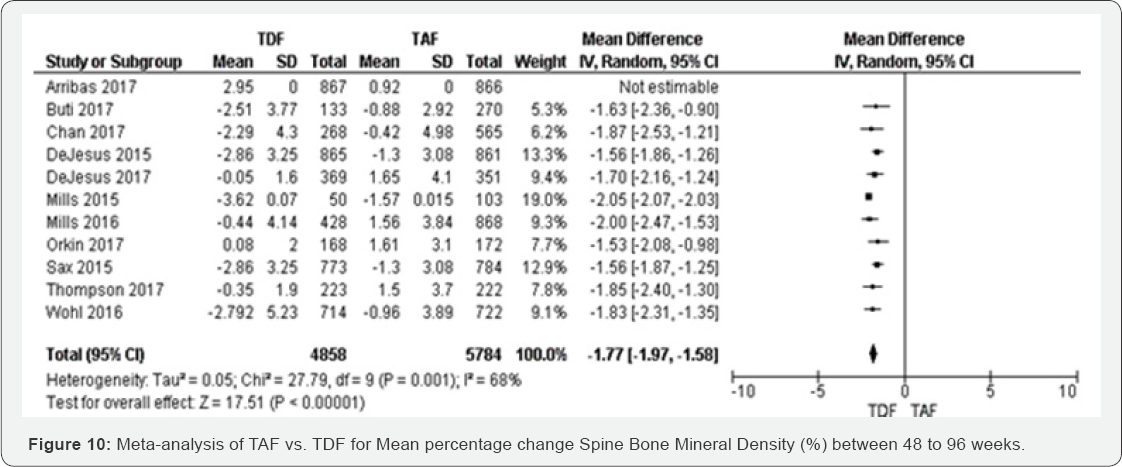
Mean percentage change Spine BMD (%) (48 to 144
weeks): Eleven RCTs were included in this meta-analysis. All of them
were statistically significant with random effect model. Transforming
from fixed to random effect, the overall results decreased to 1.6%. The
mean difference of percentage change spine BMD was decreased in TFD
compared to TAF -1.77 (-1.97 to -1.58) with P=0.001 (Figure 10).
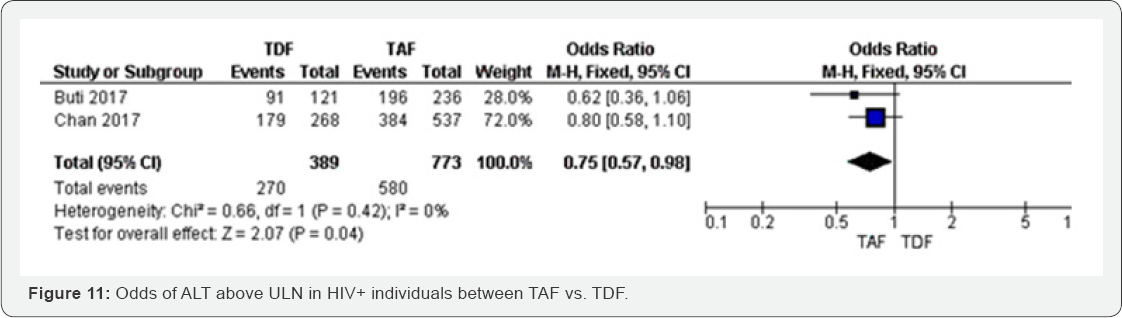
ALT above ULN (96 weeks): ALT above ULN reached the
lowest odds in TAF group compared to TDF group (OR 0.75, 0.57 to 0.98),
the two studies included in this meta-analysis were not statistically.
These results have shown moderate evidence that spine BMD is more likely
to decrease in TDF group compared to TAF group. The results were
moderately heterogeneous (I2 = 68%). Significant; however, the overall
results were statistically significant with P=0.04. The meta-analysis
was graded as high evidence. The test of heterogeneity was I2=0 (Figure 11).


Any adverse events (96 weeks): The effect of TAF
compared to TDF on any adverse events was not statistically significant
with OR 1.09 (95% CI 0.95 to 1.25, 7 studies, P=0.21), with high
evidence graded (Figure 12).
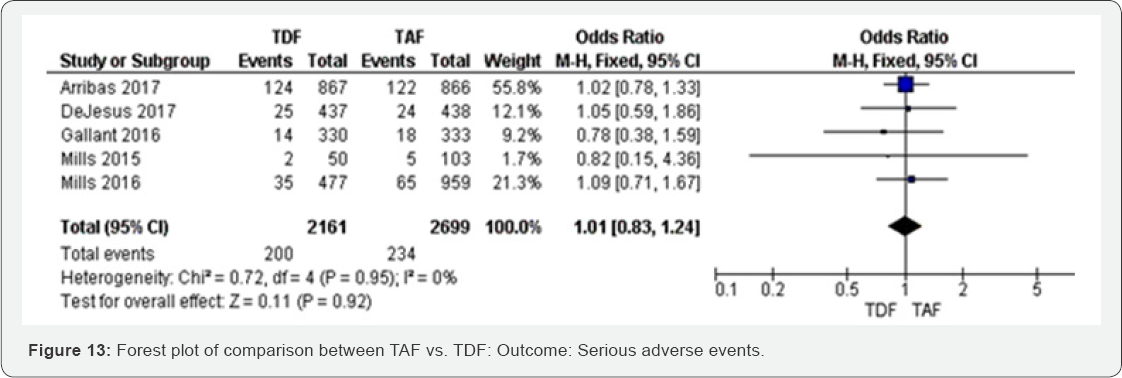
Serious adverse events (48 to 144 week): Serious
adverse events were balanced in both TAF and TDF groups. The results
with high evidence (Figure 13).
Subgroups analysis
Subgroup analyses were undertaken based on different
baseline viral load and ART regimens. The aim was to estimate a
treatment effect of different ART regimens. Two meta-analyses obtained
more 75% of heterogeneity basically Creatinine
Clearance rate (ml/min) and Mean percentage change Hip Bone Mineral
Density (%). We differentiated studies with baseline viral load less
than 50RNA/ml and those with viral above 1000RNA/ ml. Among studies,
three ART regimens were accounted: Glomerular filtration rate (ml/min) (Figure 14 &15)
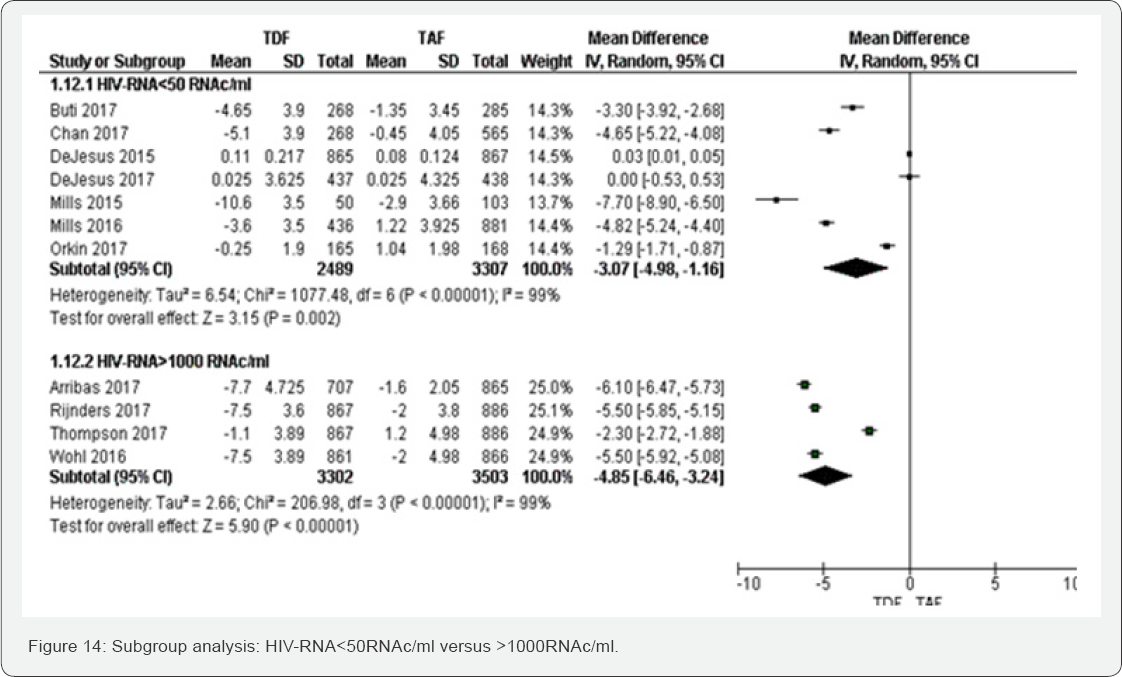
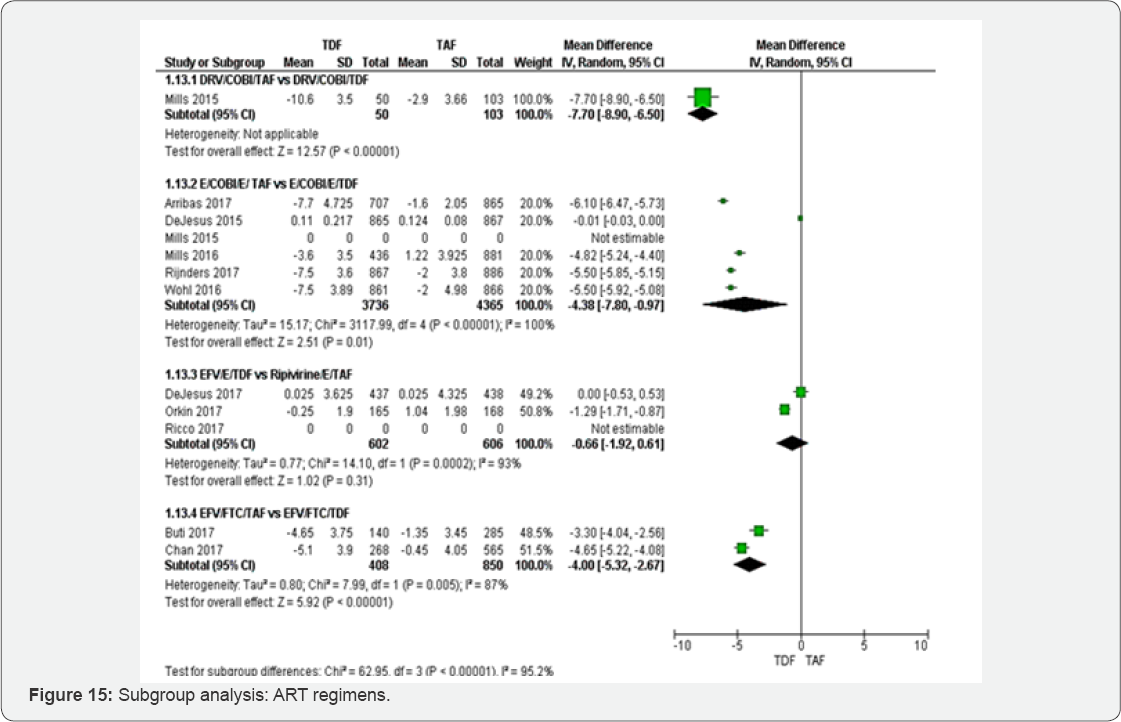
Subgroup of HIV-RNA: The test for subgroup difference
did not show any between HIV-RNA< 50 RNAc/ml to >1000 RNAc/ml.
Subgroup of different ART regimens: Subgroup analysis between different
ART regimens has illustrated that kidney injury could be more frequent
in Ripivirine/E/TDF compared to DRV/COBI/ TDF, E/COBI/E/ TDF and
EFV/FTC/TDF. DRV/COBI/TDF and
EFV/FTC/TDF subgroups have shown highly significant results.
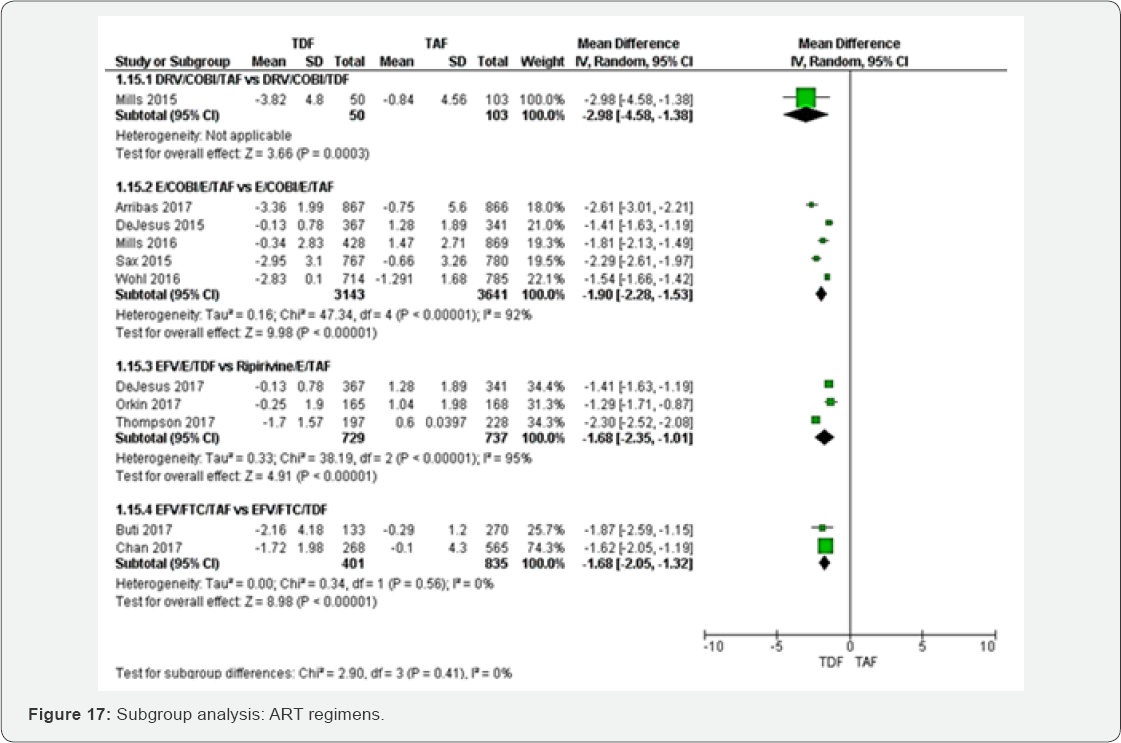
Mean percentage change Hip BMD (%)
Subgroup of HIV-RNA: The test for subgroup analysis
between HIV-RNA<50RNAc/ml to >1000RNAc/ml was not statistically
significant (P=0.10) (Figure 16).

Subgroup of different ART regimens: Subgroup analysis
between different ART regimens was not statistically significant with
P=0.41 (Figure 17).
Discussion
This systematic review has implications for patient
care, guidelines, and HIV programmes. For clinicians, TAF constitutes
the main stone of future ART regimens. These findings can inform
evidence-based guideline development and influence the WHO ART
guidelines advocating universal treatment of TAF in HIV. Our review has
limitations. We used amputation to deal with missing data. Data
extraction was amputated in three outcomes: Glomerular filtration rate
(ml/min), Mean percentage change Hip Bone Mineral Density (%) and Mean
percentage change Spine Bone Mineral Density (%). All studies were
conducted in America, Europe, Asia and Australia. We did not find any
study conducted in Sub-Saharan Africa where HIV prevalence is the
highest in the world and clinical practice has shown a significant
increase of CKDs. TAF based regimens could improve CKDs in Sub-Saharan
Africa where CKDs are rising up. Although we did not find a study in
Sub-Saharan Africa, the meta-analysis was robust enough. Then, the
evidence could still imply large implication of the study.
Concerning the HIV-RNA<50RNAc/ml from 48 to 96
weeks, there was a high evidence that TDF group was 13% less likely to
achieve VL<50RNAc/ml compared to TAF group. This result was
statistically significant with p-value of 0.02. This could imply good
clinical practice of TAF in lowering HIV-RNA. Moreover, TDF individuals
had a low MD of CD4 count (cells/^l) than TAF group (MD -18.99, 6
studies, P<00001) with high level of evidence. This means
immunological and virological parameters were well controlled with TAF.
In both TAF and TDF, there was high evidence that virological Failure
and proteinuria were balanced. Even so, the likelihood of proteinuria
was high in TDF group even if the results were not statistically
significant. The MDs of percentage change BMD was decreased in TFD
compared to TAF. This could predispose TDF group to bone injuries [34].
But the evidence was low and moderate for low hip and spine BMD
respectively. The HBV- DNA between TAF and TDF was increased to 29% with
P=0.02, showing that TAF is more beneficial than TDF in the management
of HIV/Hepatitis B coinfection. Additionally, ALT above ULN was reduced
by 25% in TAF group compared to TDF group (P=0.04). Lastly, the sides
effects were estimated the same in both TAF and TDF groups.
Statistical heterogeneity was high for summary
statistics from both Glomerular filtration rate (ml/min) and Mean
percentage change Hip Bone Mineral Density (%). We conducted subgroup
analysis to clarify the reasons of variability. Subgroup analysis has
revealed the test for subgroup difference did
not shown any difference between studies with baseline VL<50RNAc/ml
and >1000RNAc/ml. Subgroup analysis between different ART regimens
has illustrated that kidney injury could be more frequent in
Ripivirine/E/TDF compared to DRV/COBI/ TDF, E/COBI/E/ TDF and
EFV/FTC/TAF. DRV/COBI/TDF and EFV/FTC/TDF subgroups have shown highly
significant results. This subgroup analysis could influence broadly
clinical practice. In fact, DRV/COBI/TDF and EFV/FTC/TDF may be used in
high risk patients with nephrotoxic co-morbidities among which high
basal serum creatinine (Cr) level, low body weight, old age,
hypertension, diabetes mellitus and low CD4+ T cell count.
Essentially, internal consistency between results
from different analyses increases the confidence in our conclusions. In
truth, all meta-analysis included RCTs with very low risk of bias.
Random sequence generation was adequate in all RCTs (100% of studies).
Allocation concealment was well control in 39% of RCTs. 61% of them
included unclear allocation concealment. Blinding of participants and
personnel was minimized by 67% in all included studies, 27% of RCTs
revealed high risk of performance bias and 8% was unclear. Blinding of
outcome was well control in all included studies (100% low risk of
bias). Incomplete outcome data was less common, only 11% of RCTs were
high risk of bias. 22% of studies included high risk of publication
bias. However, we assessed publication bias using the funnel plots. All
the funnel plot of comparison among which HIV-RNA<50RNAc/m,
glomerular filtration rate, hip and spine BMD (%) were asymmetrical in
visual assessment. We assessed asymmetrical funnel plot using Egger's
test. Therefore, the Egger test demonstrated that HIV-RNA <50 RNA/m
was symmetrical (Egger's test=0.57, P=0.865). Other funnel plots were
asymmetric with Egger's test p-values less than 0.5. This is the main
weakness of this review. Besides, 17% of RCTs included other type of
bias.
It is important to place TAF based regimen in the
context of public health interventions in high HIV epidemic regions and
where CKDs prevalence are rising up. Our results suggest TAF contained
will reduce the pool of highly susceptible CKDs. Evidence for the
benefit of TAF over TDF in reducing HIV-RNA and HBV DNA, increasing CD4
cells, preventing CKDs and loss of bone mineral density should be
recommended in HIV or/ and Hepatitis B therapy and preventing TDF
related toxicity. The sides’ effects were balanced in both TAF and TDF
groups. Improving all those outcomes may also be beneficial in patients
with co-morbidities. Then, TAF could be used in hypertension, diabetes
mellitus and HIV co-morbidities [19-20].
Thus, scale-up of HIV therapy could lead to fewer patients developing
CKDs and HIV related co-morbidities. By the way this is likely to be one
of the most effective public health strategies to reduce TDF drug
toxicity; similarly, best way of enhancing HIV/Hepatitis B coinfection,
reducing the risk of hepatitis B complications [56-70].
Conclusion
Evidence suggests that use of TAF is more protective
and effective than either TDF. Improving renal and hepatic related
co-morbidities in HIV-infected population, TAF may be beneficial in
public health policy, specifically in high HIV epidemic regions. Based
on the results, TAF has illustrated its efficacy in all outcomes
included in this review. Findings from this study may be helpful in
preventing CKD in low and middle income countries. In reality, several
barriers are impacting in close kidneys monitoring in low income
countries. In addition, we recommend TAF based regimens in case of HIV
associated to high CKD population (Hypertension, diabetes mellitus, old
age...). Lastly, TAF contained regimens are more effective than TDF
based regimens in the management of HIV/Hepatitis B coinfection. This
review has a broad application in clinical practice. However, economic
evaluation studies should be undertaken in resource limited countries.
Acknowledgment
We sincerely thank Dr JL. Tshimwanga for reviewing
this article. Dr ANH Bulabula edited and reviewed the article. Dr LM
Muyaya and Dr JL Tamuzi critically appraised included, ongoing and
excluded studies. JL Tamuzi conceived and registered the review on
International prospective register of systematic reviews (Pospero); he
conducted electronic search, critically appraised studies, extracted
data, conducted meta-analysis, assessed the risk of bias and wrote the
review.
To know more about Open Access International
Journal of Pulmonary & Respiratory Sciences please click on: https://juniperpublishers.com/ijoprs/index.php
To know more about Open access Journals
Publishers please click on : Juniper Publishers
To know more about juniper publishers: https://juniperpublishers.business.site/
Comments
Post a Comment