A Neutrophil’s Perspective: the Innate Response to Tuberculosis Infection and the Induction of Adaptive Immunity-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS INTERNATIONAL JOURNAL OF PULMONARY & RESPIRATORY SCIENCES
Abstract
Neutrophils were traditionally viewed as short lived,
terminal, innate effector cells that eliminate microbes and remove
cellular debris at the site of infection or inflammation. They mediate
this by phagocytosis, the release of reactive oxygen species,
antimicrobial proteins and proteolytic enzymes. During recent years,
studies have demonstrated that they are longer lived than initially
thought and they mediate a large number of immune functions by the
release of a variety of preformed and newly synthesized molecules such
as cytokines and chemokines. In this review we will reconsider the
mechanism by which neutrophils operate, especially focusing on the
response to tuberculosis infection and we will look at a recent study
indicating neutrophils as sophisticated mediators of innate and adaptive
immune responses.
Immunity is defined as a host’s defence mechanism
against disease. The significance of the immune system for health is
gravely illustrated by the frequent observation of disease and infection
in individuals with inadequate or faulty immune responses. The host’s
mechanism of defence consists of innate immunity, present in all healthy
individuals as the first line of defence against infections; and
adaptive immunity, which develops more gradually and provides specific
and more specialized defence against pathogens. The importance of the
immune system for host protection against infection was intensely
highlighted during the advent of AIDS (acquired immunodeficiency
syndrome) during the 1980’s. AIDS is a spectrum disease caused by an
infection with HIV (human immunodeficiency virus) [1]. As the disease
progresses, the immune system steadily declines, resulting in an
increased susceptibility to opportunistic infections that can become
life threatening; the reactivation of latent infections such as
tuberculosis; and greater incidence of several cancers. In individuals
with a healthy immune system, latent tuberculosis are not eradicated but
are constrained by intact immune responses. Tuberculosis is caused by
infection of Mycobacterium tuberculosis (M. tb), an
intracellular bacterium [2]. According to the World Health Organization
during 2013 tuberculosis accounted for about 1.3 million deaths on
average [3]. Today, one of the most prominent threats in the abolishment
of the tuberculosis epidemic is multidrug resistant M. tb
(MDR-TB), which developed due to extensive and uncontrolled use of
antibiotics. It is evident from these statistics that more effective
tuberculosis treatments and diagnostic tools are required.
Neutrophils, being the specialized front-line
fighters, arrive at the scene within minutes after a breach of immunity.
They are directly associated with inflammation and react vigorously
against pathogenic infection, often leaving behind a trail of
immunopathology. Neutrophils instruct monocytes, dendritic cells and
other lymphocytes and aid as a direct connection between innate and
humoral immune responses. In this review we reassess the duty of the
innate immune system, especially focusing on the role neutrophils play
during tuberculosis infection. We will also consider a pioneering study
led by Andrea Cerutti et al. [4] in uncovering novel communications
between different divisions of the immune system.
Neutrophils are effector cells that form part of the
innate immune system. They are also known as polymorphonuclear
neutrophils (PMN’s) due to their lobe shaped nuclei. Together with
eosinophils and basophils they form part of the granulocyte cell family.
The cytoplasmic granules contained within
neutrophils are characterized by their ability to not take on
basophilic (blue) or acidophilic (red) dye stains, they instead
colour pale pink during blood smear stains [4]. These native
myeloid cells are formed in the bone marrow where growth
factors and cytokines instruct pluripotent hematopoietic
cells to differentiate into myeloblasts. These myeloblasts are
mouldable cell types dedicated to develop into granulocytes.
During neutrophil development, protein-containing cytoplasmic
granules are formed and released into circulation following
their maturation [4]. The strictly monitored process by which
matured neutrophils are released from the bone marrow are
regulated by cytokines and chemokines. Stimulation to release
neutrophils into circulation (from the bone marrow) is governed
by the SDF-1 α/CXCR4 chemokine axis, which also maintains
an assemblage of neutrophils to promote rapid release should
an infection arise [5]. Between 50-70% of the white blood cell
population is represented by neutrophils, making them the most
abundant white blood cell type. They are highly mobile and
found dispersed in tissues, but are predominantly found in areas
of acute inflammation and severe necrosis.
Neutrophils are the first line of defence against infection
and migrate to the site of inflammation or tissue damage within
minutes following trauma. These innate immune cells are the
first to be activated and a key attribute of acute inflammation
[6]. Neutrophils undergo a process of degranulation following
activation and release into circulation. During this process an
extensive amount of membrane delineated granules release
their payload consisting of potent anti-microbial agents, such
as alkaline phosphatase- containing granules, specific granules
and azurophil granules. Azurophil granules include ionic granule
proteins such as “lysosomal enzymes” and defensins. Defensins
are antimicrobial peptides capable of inserting themselves
into microbial cell membranes via electrostatic interactions
[7] or through transmembrane potential driven insertions
[8]. The membrane insertion brings about a change in the
permeability of the membrane, and ultimately results in demise
of the microbe. Defensins are effective against a wide array of
organisms including bacteria, fungi and even viruses [9]. These
anti-microbial peptides further add to the immune response by
scrupulously inducing the migration of CD4+/CD45RA and CD8+
T-cells in humans and also serve as a chemo tactic for immature
dendritic cells derived from either peripheral blood monocytes
or CD34+ progenitors [10].
Neutrophils also have the ability to produce toxic oxygen
species that include hydrogen peroxide, hydroxyl radicals and
superoxide anions. These reactive oxygen species (ROS) have
numerous functions, including acting as cellular messengers
[11], regulating the apoptotic process of neutrophils [12] and
modulating other reactive immune cells [13]. The microbicidal
role of oxygen derived free radicals are emphasized by theirability to promote lipid peroxidation, DNA damage and the
oxidation of proteins, resulting in cell death [14].
Even though neutrophils are the chief motive of the innate
immune system, in humans they only spend an average of 5 and
half days in circulation [15]. The brief lifespan of neutrophils
compared to other innate immune cells, could be attributed to
their arsenal of anti-microbial compounds that could induce
severe immunopathology and cause serious harm to the host
if released unrestrained. Constitutive apoptosis is another
mechanism used to control neutrophil numbers, to regulate the
inflammatory potential of these innate cells [16]. This essential
process maintains the delicate balance between neutrophils
behaving as effectors during host defence and neutrophils
functioning as inducers of immunopathology. Abadie and
colleagues [17] infected mice with a genetically modified strain of
Mycobacterium bovis bacilli Calmette-Guerin (BCG) that express
an enhanced green fluorescent protein (EGFP). Co-expression of
EGFP enabled them to determine the essential role neutrophils
play in the capture and transport of rBCG-egfp to the secondary
lymphoid organs, including Peyer’s patches, lymph nodes and
spleen. Their results showed that neutrophils can also play a
part in antigen presentation in vivo. The conclusion was that
neutrophils have the capacity to exit the site of infection via the
afferent lymphatic system, migrate to the secondary lymphoid
tissue and take part in the transport and presentation of live
microbes [17].
The release of cytokines and chemokines recruit neutrophils
to the site of infection. After an encounter and subsequent
infection by M. tb, macrophages produce interleukin-8 (CXCL8
or IL-8) [18]. With regards to neutrophil immunity, CXCL8 is one
of the most influential chemokines [19]. Neutrophils possess
a high number of chemokine receptors that are specific for
CXCL8. It serves as a chemo attractant and potent angiogenic
factor, crucial for activation and recruitment of neutrophils [19].
A significant correlation exists between the amount of CXCL8
protein present and the number of neutrophils accounted for
[20]. Activated human CD4+ T-cells secrete interleukin-17 (IL-
17). The cytokine, IL-17 is responsible for inducing an elevated
concentration and an increased release of CXCL8 from human
bronchial epithelial and venous endothelial cells. It was further
illustrated that in vivo, after intra-tracheal addition of hIL-17
(human interleukin-17), neutrophils were selectively recruited
to the airways of the rats. Laan et al. [21] established that there
exists a link between neutrophil recruitment and T-lymphocytes
by demonstrating that hIL-17 mobilizes neutrophils to the site of
infection via the release of CXC chemokines [21].
During infection neutrophils move to the area of infection
where they attempt to kill the intruding micro-organism by
phagocytosis followed by exposure to ROS and other antimicrobial
metabolites. The measure of resistance that the hostwill have against various bacterial and fungal infections is also
determined by neutrophils. Elevated chemotaxis accompanied
by an increased accumulation of neutrophils in the granuloma
suggests another role of these innate cells during M. tb infection.
By activating DC’s (dendritic cells), neutrophils also act as a
messenger between the innate and specific acquired immune
system [22]. Neutrophils have also shown to enhance immunity.
When apoptotic neutrophils, infected with mycobacteria,
are phagocytosed by macrophages, the acquired neutrophil
granules add to the increased microbicidal effect that these
macrophages have against the bacteria [19,21]. After inhalation
of M. tb, neutrophils and macrophages are of the first cells that
come into contact with the bacteria. Macrophages, being a
substantial source of CXCL8, are responsible for the increased
recruitment of neutrophils to the site of infection. As a result,
the newly recruited neutrophils produce cytokines like TNF-α
(tumour necrosis factor-alpha) which have a paracrine effect on
macrophages [19]. This illustrates that the immune system is
interlinked in function, and how various components influence
one another to shape the type of immune response that is
elicited [23].
Because of their notorious association with immunopathology
recognized during acute infection, most data pertaining to
neutrophils aim attention at the intracellular killing mechanism
and overlook their probable extracellular activities. In recent
years [24], described an extracellular, neutrophil-mediated antimicrobial
mechanism to contain and kill micro-organisms. This
mechanism involves the formation of neutrophil extracellular
traps (NETs), as shown in Figure 1, which consist of chromatin,
lined with anti-microbial proteins. These proteins are granular
in nature and have the ability to contain and kilo gram-positive
bacteria [25,26], gram negative bacteria [27] and even fungi
[28]. NADPH oxidase, coupled with NET formation, produce ROS
responsible for induction of a cell death process unique from
necrosis or apoptosis.
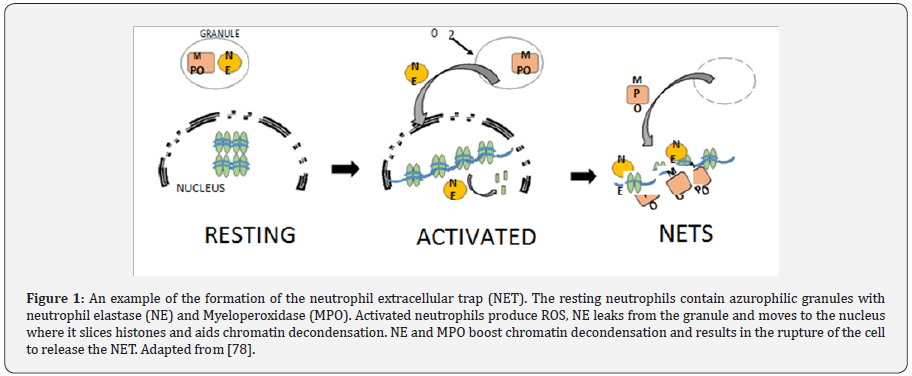
Neutrophils remain dormant in circulation until they
encounter an infectious agent, this is followed by a phase of
activation that promotes and enhances inflammatory responses
and anti-microbial action [29]. This phase of activation includes
various stages of phenotypical and functional changes in
neutrophils. The cell surface receptors employed by neutrophils
in the interaction with microbes are altered by low levels of
activating agents. Direct recognition, as well as opsonisation,
the process by which opsonins coat infecting bacteria making
them more prone to be phagocytosed, seems to play a key role
in the process of mycobacterial internalization. Alemán and
colleagues [30] showed that mice deficient in Toll like receptor
2 (TLR2) had reduced control over both M. avium and M. tb
infections, highlighting a role for TLR2. Toll-like receptor 4
(TLR4) should not be neglected, as Godaly [31] illustrated that
when TRL4 was blocked, CXCL8 production in response to BCG
infection was significantly reduced. Bacteria are phagocytosed
once recognition has taken place. Neutrophils play a central role
in phagocytosis, the primary mechanism by which pathogens
and cell debris are removed from the body. Phagocytosis is a
dynamic process mediated by cell receptors. The internalization
of microbes occurs through the cell membrane into vacuoles
called phagosomes. Inside these vacuoles, microbes are exposed
to various anti-microbial peptides and degradative molecules.
The type of interaction between the microbe and the neutrophil
determine the specific mechanism of internalization that is
utilized. As soon as neutrophils exit the circulatory system and
enter the site of infection, they interact with invading pathogens
and become activated. They are able to partake in specific antimicrobial
actions upon stimulation by certain chemokines and
cytokines.
With their rapid action against infection, it is evident that
neutrophils play an essential part in creating the optimal
environment for the host to reciprocate with a suitable adaptiveimmune response. This is accomplished by using chemokines
and cytokines as mediators to issue instructions to virtually
all other types of immune cells. This is a critical process for
the development of an appropriate inflammatory response.
Neutrophils maintain a low transcriptional signature during
their inactive state in the circulatory blood and only once
they encounter infection do they experience an immense
transcriptional burst and successive activation which results in
the formation of signaling compounds [32,33].
Even though, compared to other immune cells, neutrophils
do not produce a large amount of cytokines per cell, at the site of
infection or inflammation they are plentiful and present in large
numbers, making their relative contribution rather significant
[34]. Since the primary response of these innate cells is to boost
their numbers, CXCL8 is most abundantly produced due to its
main function being to recruit more neutrophils [35]. In addition
to chemokines and cytokines, a variety of other signaling
compounds are secreted by neutrophils. These include granule
content [36], lipids [37], hydrogen peroxide (ROS) and some
mediators by means of cell to cell contact [38]. Other leukocytes,
such as macrophages, cooperate with neutrophils to combat
various pathogenic infections. Using immunohistochemistry,
Ramos-Kichik et al. [24] noticed that following a mycobacterial
infection, macrophages contained granulocytes. This
adequately illustrated that through phagocytosis, macrophages
obtained lactoferin, a protein produced by granulocytes such
as neutrophils and inherently not present in macrophages.
Previously, the presence of neutrophils during mycobacterial
infections was thought of as inconsequential and temporary,
however, these findings significantly highlighted the function
of these leukocytes [23]. Dendritic cells (DCs), classified as
probably the most important antigen-presenting cells, have the capacity to capture and present antigens in the secondary
lymphoid tissue. They also release interleukin-12 (IL-12) which
has been demonstrated to be integral in the stimulation of a T
helper 1 (Th1) directed cytokine response [39]. Van Gisbergen
et al. [38] confirmed that active neutrophils, both in vitro and in
vivo, robustly cluster with and activate the maturation of DCs.
This facilitates them to set off a strong T-cell response directed
at a type 1 T-cell polarization. This DC-neutrophil interaction is
aided by the binding of C-type lectin unique to DCs (DC-SIGN), to
Mac-1 [38]. The interaction of immature DCs with neutrophils
may, in distant lymph nodes, modulates immune responses.
Bennouna et al. [40] confirmed this with data from a murine
study, showing that both DC maturation and cytokine production
was induced by neutrophil derived TNF-α. Neutrophils have also
been shown to, in vitro, collaborate with natural killer (NK) cells
and DCs. The study by Costantini et al. [41] f confirmed that
neutrophils, using CD18-ICAM-1 interactions, very specifically
communicate with DCs. This correspondence promotes the
production of IL-12p70 by DCs, which in turn, results in the
stimulation of Interferon-γ (INF-γ) production by NK cells and
eventually furthers the activation of neutrophils, culminating
in a positive feedback loop. Simultaneously, by direct binding
(in a cell to cell manner), NK cells become further activated by
neutrophils [41].
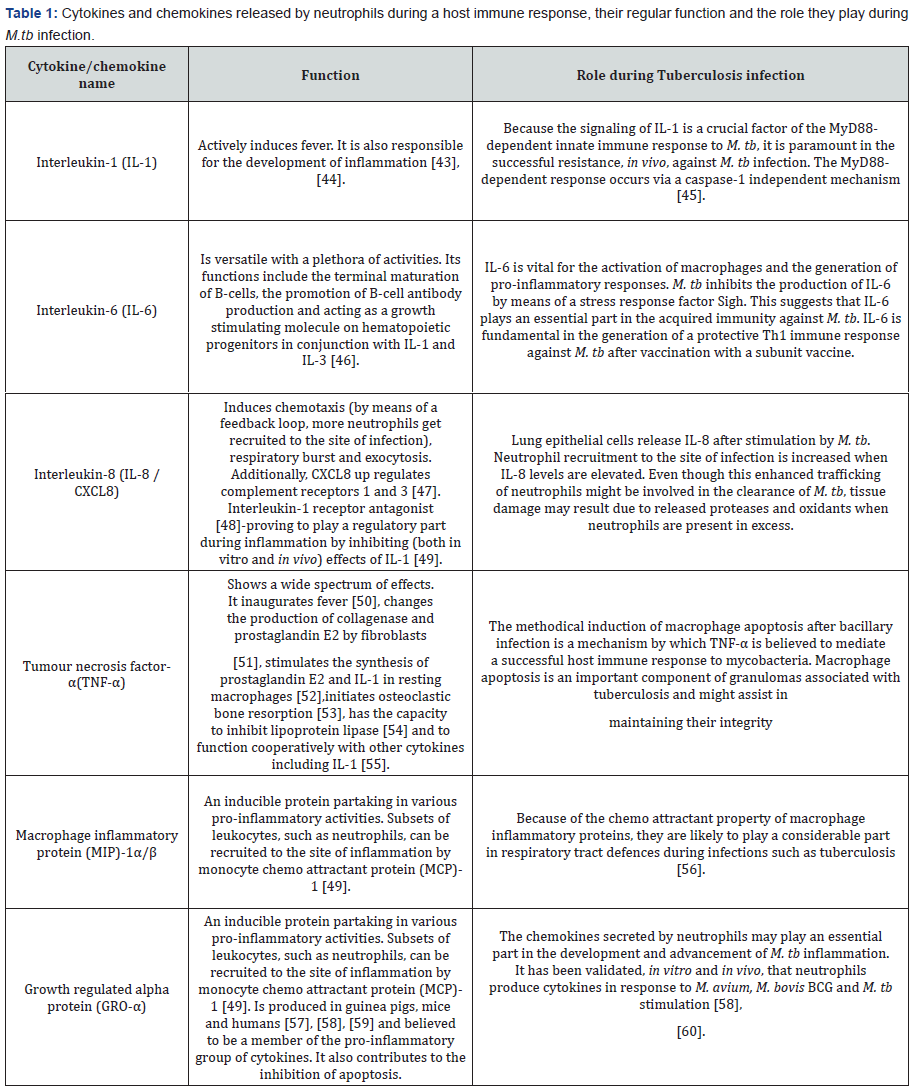
By means of cytokine and pattern-recognition receptors,
neutrophils may be directly activated to secrete immunomodulatory
elements [42]. Surprisingly, even cathelicidins and
defensins (anti-microbial peptides found inside the granules
of neutrophils) have the capacity to be immune-modulatory.
Table 1 outlines the function of some of this immune response
modulating cytokines.
Collaboration between the innate and adaptive arms of
the immune system is required for the successful eradication
of pathogens. While the innate branch launches a rapid, less
specified response against pathogens by recognizing conserved
microbial patterns, the adaptive response is highly specific
and somewhat delayed, taking days to become apparent. The
participation of innate immune cells in the mediation of B-cell
responses has been generally restricted to opsonisation and
phagocytosis of pathogens coated with antigens. However,
studies by Chen et al. [61] and Chu et al. [62] illustrated that
innate immune cells, basophils and eosinophils respectively,
secrete factors such as interleukin-6 (IL-6), a proliferationinducing
ligand (APRIL), B-cell-activating factor of the TNF
family (BAFF), which stimulate the activation of B-cells. Similarly,
it has been shown that neutrophils impact adaptive immune
responses during infection by regulating the activation of
dendritic cells by way of interleukin-10 [63] and alarmins [36].
However, it has been largely unknown how neutrophils regulate
a response in the humoral branch of the adaptive immune
system. In a pioneering study by Andrea Cerutti, the authors
demonstrated that splenic neutrophils have the capacity to act
as proficient helper cells for splenic marginal zone (MZ) B-cells,
resulting in the generation of matured antibodies with increased
affinity for a specific antigen (fully illustrated in Figure 2. The
study commenced by analyzing neutrophil distribution in tissue
sections taken from peripheral lymphoid organs of individuals
free from infection or inflammation. They observed in regions
neighboring the splenic MZ that neutrophils were found in
abundance. The aforementioned distribution is observed in
both mice and macaques, which implied that these neutrophils
around the MZ might be consequential in the maintenance of
homeostasis. Moreover, in pathological spleens this distribution
changes, such that neutrophils penetrate the germinal centres
and follicular mantle. The confinement of neutrophils to the
area around the MZ signifies that they are in an optimal location
to react to circulating antigens and also leave them in adjacent
to MZ B-cells. These B-cells are usually linked with antibody
responses that are T-cell independent. In light of this, the authors
showed that splenic neutrophils differ from those in circulation
in such that in MZ B-cells they are able to moderate activation of IgM secretion. Subsequently these cells were termed B-helper
neutrophils (NBH), and an in depth analysis of this population
unveiled the possible molecular mechanism by which they
regulate MZ B-cell activation. Compared to general circulating
neutrophils, expression of B-cell stimulating molecules such
as APRIL, BAFF, Interleukin-21 and CD40L, are significantly up
regulated in NBH. Furthermore, activation of MZ B-cells can occur
in medium conditioned with NBH cells, an effect that is annulled
when signaling is blocked through these receptors. However,
activation of MZ B-cells seems to be influenced by contact–
dependent mechanisms as well since greater antibody secretion
is observed after incubation with NBH cells. Interestingly,
unlike general circulating neutrophils, the NBH community
impulsively forms neutrophil extracellular trap (NET) like
projections containing DNA. Lead better and colleagues [64]
proposed that NETs might serve as a potential source of toll-like
receptor 9 ligand containing immune complexes, which might
facilitate activation of B-cells. Nevertheless, the identification
of a NBH cell population (able to acts as proficient helper cells
for specifically MZ B-cells) unveils an intriguing new avenue for
the correspondence between the adaptive and innate immune
branches.

How is the MZ B-cell population affected by this NBHmediated
support? The activation of follicular B-cells following
T-cell dependent antigen presentation is generally partnered
with the development of germinal centre’s, this has been
adequately described [65]. Germinal centre’s have commonly
been associated with the process of somatic hyper mutation
(SHM) which generates a plethora of Ig genes and results in an
assemblage of high-affinity clones as well as the development of
immunological memory. Nonetheless, even though it has been
shown that during a systemic infection, CD11c(lo) dendritic cells
encourage the development of IgM-secreting plasma blasts from
MZ B-cells [49], considerably less is known about the influence
of helper cell assistance on the initiation of T-cell independent
immune responses. This study by Puga et al. [4] demonstrated
that the expression of transcription factors such as XBP1 and
Blimp1 as well as surface marker CD38 in MZ B-cells, is triggered
by NBH cells. The expression of these factors is an indication of
the formation of plasma blasts. Moreover, as expression of AID (a DNA-editing enzyme necessary for class-switch recombination
and somatic hyper mutation) is up regulated in MZ B-cells in
the vicinity of NBH cells, class switching was shown to have
occurred in secreted antibodies, with generation of IgA and
IgG2 being favored. Notably, even though in individuals with
severe congenital neutropenia (abnormally low neutrophil
count), levels of class-switched antibodies in response to
T-cell dependent antigens were normal. Decreased levels of
IgG and IgA in response to T-cell independent antigens, like
lipopolysaccharides (LPS) were observed. Sequencing indicated
that, at least in humans, NBH-activated MZ B-cell secreted
antibodies acquire mutations similar to those observed during
somatic hypermutation. Interestingly, the assistance from NBHcells
seem to trigger antibody expansion from MZ B-cells, this
effect is similar to that of CD4+ T-cells on follicular B-cells.
The origin of these NBH cells comes into question, given their
ability to mediate class-switched antibody secretion from MZ
B-cells. When general circulating neutrophils are exposed
to interleukin-10, they become inducible NBH-like cells and
expression of APRIL and BAFF is up regulated. STAT3 and
JAK2 signaling is required for the generation of this inducible
population. Sinusoidal epithelial cells, in response to microbial
peptides, secrete a variety of neutrophil-attracting chemokines
as well as interleukin-10. These sinusoidal epithelial cells are
found close to NBH cells in the splenic MZ. In light of this, Puga et
al. [4] postulated that microbial ligands, entering the circulation
by translocation across microbial surfaces [66], prompt both
chemotactic signals to and reprogramming of circulating
neutrophils which result in the generation of a NBH population.
In agreement with this, splenic NBH cells are established early
in fetal life, but only about two days after birth is this population
significantly enhanced, this event coincides with the bacterial
colonization of mucosal surfaces. Furthermore, mice that are
either unable to generate toll-like receptor signaling or are born
germ free, have a decreased NBH population. These observations
suggest that given a healthy and functional immune system, NBH
cells stimulate the formation of class-switched antibodies from
MZ B-cells in response to T-cell independent microbial antigens
under steady state conditions.
Various studies show definitive evidence that neutrophils
are essential to the protective immune response against M.tb
infection [17,57,67]. Martineau and colleagues [68] provided
evidence that neutropenia led to a considerable decline in
both BCG and M. tb levels in whole blood. Numerous studies
adequately showed that neutrophils play a major role in the
immunopathology during pulmonary tuberculosis infection,
with some stating that they are noxious to the host’s control
of the mycobacterial infection [54-56]. Another study showed
that following intratracheal infection, a TB susceptible mouse
strain had surprisingly high levels of neutrophils accumulating
in the lungs for extended periods [69]. In comparison to less
susceptible strains, they demonstrated prolonged existence andlower expression of the CD95 apoptotic receptor associated with
greater mobility and phagocytic proficiency of M. tb. The study
concluded that the development of immunopathology during
tuberculosis infection was driven by the above mentioned
features as well as the fact that/ compared to macrophages,
neutrophils battle to control mycobacterial growth [70]. In
recent years it became apparent that neutrophils are the main
cells infected with actively replicating mycobacteria [71]. This
adds to the intricacy of the part they play in M.tb infection,
indicating that they act as a concealed stratagem during host
infection with M. tb.
Neutrophils have a broad spectrum of anti-microbial actions.
One such action involves the use of human neutrophil peptides
(HNPs) which form part of the defensin family of anti-microbial
proteins [72]. Mice infected with M. tb H37Rv have shown a
significant reduction in the bacillary load in liver spleen and
lungs after time and dose dependent treatment with HNP-1
[73]. Martineau et al. [53] also illustrated that HNPs 1-3 kill
M. tbin microbiological media. Neutrophils produce lipocalin
2 and cathelicidin LL-37, these peptides both have the ability
to restrict mycobacterial growth, with lipocalin 2 acting in
an iron dependent manner [74]. This evidence indicates that
neutrophils play a considerable part in the host defence during
innate immunity. Antimicrobial peptide production facilitates
this defense.
During inflammation neutrophils actively produce and
secrete a serine proteinase known as ELA2 or leukocyte elastase.
It forms part of the chymotrypsin family and has significant
microbicidal activity by killing target bacteria and destroying
host tissue [75]. ELA2 comprises of three amino acid residues;
histidine, aspartate and serine, which are involved in a charge,
relay system. This charge relay system allows for the proteinase
activity. Within the primary polypeptide these residues are
dispersed throughout, it is only once the protein has folded
and its three dimensional conformation is complete, that
these residues form a triad capable of proteinase activity [76].
Azurophil granules contain ELA2. The function of this elastase
is to hydrolyse wide variety of proteins inside the azurophil
granules as well as proteins in the extracellular matrix, following
its release from the azurophil granules. Neutrophil elastase kills
gram-negative bacteria [77], facilitates NET production [78]
and degrades bacterial virulence factors [77]. When activated,
neutrophil elastase translocates to the nucleus where it partially
digests certain histones, promoting chromatin decondensation
and leading to NET formation [79]. Myeloperoxidase (MPO), in
the presence of ROS such as hydrogen peroxide, had persistent
microbicidal action against M.tb H37Rv [79]. When activated,
granulocytes such as neutrophils, utilize the myeloperoxidase-
H2O2-Cl- system to generate reactive aldehydes [80] which at
the site of inflammation, covalently alter both membranous
and soluble proteins of cells [81]. Also, MPO acts in conjunction
with neutrophil elastase to independently drive chromatin
decondensation from the elastase’s enzymatic activity [78].Lactoferrin, a multifunctional immune protein present in a range
of secretory fluids is also contained within the secondary granules
of polymorphonuclear leukocytes such as neutrophils [82]. A
further study [83] demonstrated the antibacterial capabilities
of human lactoferrin early on. This is achieved by lactoferrin
sequestering iron from the environment, making this vital
element unattainable to potential pathogens [84]. Lactoferrin
functions as an adjunct adjuvant in BCG vaccine efficacy and this
leads to a boost in protection against future trials with virulent
M. tb. An increase in the production of IL-12(p40) as well as an
increase in relative ratios of IL-12/IL-10 was observed in mice
after a single immunization with lactoferrin [84], this would,
in turn, result in the increased recruitment and production of
neutrophils.
Neutrophils extracellular traps (NETs) also demonstrate a
unique mechanism for the control of infections with intracellular
mycobacteria. The formation of NETs is brought about by
cellular changes induced by M. tb, resulting in neutrophil death
and subsequent release of M. tb [24]. Even though NETs are able
to capture mycobacteria after their release, they are not able to
eradicate them. In vivo mycobacterial control could be facilitated
by the trapping of M. tb by NETs thus restricting distribution of
mycobacteria and limiting the infection to the local environment
only. A different function for the NET-mediated trapping of
M .tb is presumably the sequestering of local chemokines and
cytokines, thus initiating granuloma development by promoting
recruitment of other phagocytes. Since only a proportion of
neutrophils undergo NET formation, the remaining neutrophils
are believed to play a role in phagocytosis and other neutrophilic
obligations [24]. Even though reactive oxygen species are
produced, the combined action with NETs does not eradicate
mycobacteria as successful as other microbes [24,85]. It is
suggested that NETs bind to the electron dense layer on the
outermost structure of mycobacteria. This structure is composed
of polysaccharides with their negatively charged groups exposed
[86,87].
Neutrophils are capable of undergoing apoptosis [12,13,58].
After phagocytosis by neutrophils, mycobacteria are exposed
to a variety of antibacterial substances within the intracellular
environment of the neutrophil. If these bactericidal substances
fail to successfully eradicate the microbe, the neutrophil induces
apoptosis in a final effort to eliminate the phagocytosed bacteria.
It has also been proposed that in an attempt to be spared
from the immune response, mycobacteria induce apoptosis in
neutrophils after infection [88-91].
TNF-α is one cytokine responsible for inducing apoptosis in
neutrophils [92]. This was illustrated in vitro [93] that neutrophils
stimulated with TNF-α and bacterial lipopolysaccharide
(LPS) resulted in apoptosis. The study also suggested that
apoptosis might be the cause for the low viability observed
with neutrophils, and that the obliteration of these neutrophils,
unsuccessful in their attempt to kill the mycobacteria, tips thescale towards the infiltration of mononuclear cells rather than
neutrophils, during the alteration of the inflammatory immune
response against M. tbinfection. They also proposed that the
rapid cell death of neutrophils at the site of infection might be
attributed to M. tb [94].
Neutrophils are traditionally convicted of being destructive,
they arrive on the scene too early, in colossal numbers, and their
reaction is unmerciful and usually leads to immunopathology. As
more discoveries are made regarding these innate immune cells,
however, neutrophils are beginning to emerge as influential
mediators between innate and adaptive immune branches and
crucial for an effective immune response against pathogens. They
function as effectors with a plethora of cytotoxic constituents at
their disposal, as well as preparing the micro environment for
the more specific adaptive response by secreting the required
chemokines and cytokines. They instruct monocytes, dendritic
cells and other lymphocytes and serve as a direct connection
between innate and humoral immune responses. They greatly
influence the decision to initiate, alter or maintain a specific
immune response. Being the devoted front line fighters that
they are, they fight in life and even in death and come prepared
with an arsenal of weaponry against pathogens such as M. tb,
employ kamikaze tactics by apoptosis and form NETs through
specialized cell death.
Uncovering the full complexity of the mechanism by which
neutrophils operate proves to remain a challenge. Unanswered
questions that still remain include:
- The source of the initial signal that initiates formation of the NBH population and
- The specific regulatory mechanism by which NBHmediated MZ B-cells are activated. Answers to these questions could lead to potential therapeutic advances in the enhancement of basal immunity by the calculated manipulation of neutrophils.
To know more about Open Access International
Journal of Pulmonary & Respiratory Sciences please click on: https://juniperpublishers.com/ijoprs/index.php

Comments
Post a Comment