Tuberculosis: Basic and Clinical Relevant Aspects-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS INTERNATIONAL JOURNAL OF PULMONARY & RESPIRATORY SCIENCES
Abstract
Tuberculosis remains a major public health problem
around the world. This disease is caused by M. tuberculosis. The World
Health Organization (WHO) estimated 10.4 million new tuberculosis cases
worldwide in 2015 and 1.4 million of deaths in the same period. Besides,
an estimated of 2 billion people worldwide have latent tuberculosis
infection, from which 10% will develop tuberculosis disease.
The emergence of antibiotic-resistant strains
associated to more aggressive clinical entities is a challenge in
tuberculosis treatment. It is estimated that, in 2010, about 650,000
tuberculosis cases were resistant to isoniazid and rifampicin. This
situation forces governments to maintain a permanent surveillance of the
sensitivity in new bacterial isolations.
In this review we discuss tuberculosis generalities,
from the general description of the pathogen and its epidemiological
effect to clinical entities, diagnosis, and treatment associated with
the infection.
Abbreviations: AAFB: Acid Alcohol Fast Bacilli; AIDS: Acquired Immune Deficiency Syndrome; BCG: Bacillus Calmette-Guérin; CDC: Centre for Disease Control; CNS: Central Nervous System; Cyd: Cytochrome bd oxidase; DNA: Deoxy Ribonucleic Acid; ELISA: Enzyme-Linked Immunosorbent Assay; GM-CSF: Macrophage Colony Stimulating Factor; HIV: Human Immunodeficiency Virus; LAM: Lipoarabinomannan; MDR: Multidrug Resistant Strain; PCR: Polymerase Chain Reaction; PET-CT: Positon-Emission Tomography with Computed Tomography; PPD: Purified Protein Derivate; rBCG: Recombinant BCG Strains; TLR: Toll-Like Receptors; WHO: World Health Organization; XDR: Extensively Resistant Strain
Tuberculosis is a global problem of public health.
Only in 2015, the World Health Organization (WHO) estimated 10.4 million
new incident cases worldwide (Figure 1), mostly in India, China,
Nigeria, Pakistan, and South Africa and 1.4 million
tuberculosis-associated deaths during the same year [1]. Additionally,
there were an estimated 100 cases per 100,000 habitants/year of
tuberculosis incidence worldwide [2]. Among the tuberculosis-related
deaths, more than 95% occur in low and medium income countries, being
tuberculosis the principal cause related with patients infected with the
human immunodeficiency virus (HIV) [3]. In fact, the WHO reported that
almost 1.2 million incident tuberculosis TB cases subjects were
co-infected with HIV by 2015 [1]. Furthermore, it is estimated that 2
billion people worldwide are latently infected by the tuberculosis
bacterium [4,5]. Among them, nearly 10% will develop some presentation
of the disease [6].

Tuberculosis is an old disease, which pathogen has evolved
together with the human [7]. This was evident in a Turkish
study that identified a 500,000-year old Homo erectus fossil
with typical lesions of tuberculosis [8]. However, it has been
estimated the existence of an early progenitor of Mycobacterium
tuberculosis 3 million years ago in East Africa, and it is possible
that all members of the Mycobacterium tuberculosis complex
share a common African ancestor 35,000-15,000 years ago
[9]. Despite its antique, the first’s descriptions of this disease
were done by Hippocrates, Paracelsus and Galen and then, in
the classical Greece, the tuberculosis was a well-recognized
pathology under name of phthisis [9]. To 1882, Robert Koch
identified Bacterium tuberculosis as causal agent of tuberculosis
[10]. After that in 1896, Lehmann and Neumann named it
Mycobacterium tuberculosis [11].
Among the M. tuberculosis strains that infect the human,
there are two “antique strains” that remain in Africa, while
the remaining strains are spread in the rest of the world [8].
Recently, new M. tuberculosis strains have emerged, which are
resistant to the election antibiotics and they are associated with
a more aggressive clinical presentation. In 2010, it is estimated
that approximately 650,000 tuberculosis cases were resistant to
the first-line drugs (isoniazid and rifampicin) [12].
The WHO plans to significantly reduce both the incidence
and death associated with tuberculosis between the years 2015
and 2030 [13]. To achieve this goal, several strategies have been
designed; they include development of: new vaccines, quick
diagnostic tests, and better treatments schemes [8,11]. Regardless
the knowledge of the risk factors related to tuberculosis and its
evolution to the active disease (including poverty, overcrowding,
malnutrition, alcoholism, HIV infection, silicosis, chronic kidney
failure, diabetes, smoking, and immunosuppressive therapy
[6]), we still lack information on how this predisposition factors
interact to activate the disease in each patient [14].
Tuberculosis is caused by a group of phylo genetically
related bacteria known as Mycobacterium tuberculosis complex
[15]. This complex includes M. tuberculosis, M. bovis, M. bovis
(BCG), M. africanum, M. microti, and M. canettii, which share
more than 99% of identity at nucleotide level [16]. In humans,
the tuberculosis disease is usually caused by M. tuberculosis and
M. africanumstrains, bothobligate human pathogens without
known animal reservoir [17] and with limited survival time
outside the host [16]. Besides, the M. bovis strain has been
related to human infections, particularly in a zoonotic way with
unpasteurized milk as a source [1].
M. tuberculosisis an intracellular bacterium that lives and
duplicates inside the phagosome of macrophages, this bacteria
may also initially infect type II pneumocytes in the pulmonary
alveoli [18].
The Mycobacterium bacillus is slightly curved,
with a size
close to 1-4μm in its major axis and 0.3-0.6μm in its minor axis, while
its replication time is 12-24 hours [19]. It is poorly Gram(+ve),
aerobic, immotile, and of slow growing; it is not a sporeforming
bacterium and is characterized by a cellular wall that is
rich in α-acetylated and β-hydroxylated long-chain fatty acids,
mostly mycolic acids [17,20,21] (Figure 2).
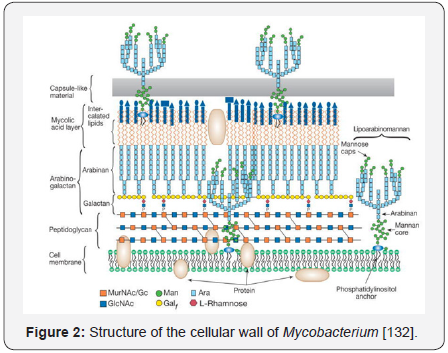
Among other functions, the cellular wall confers to
mycobacteria resistance to antibiotics, acid and alkaline
environments, and intrinsic resistance to desiccation. The cellular
wall composition also confers an extreme hydro phobicity to the
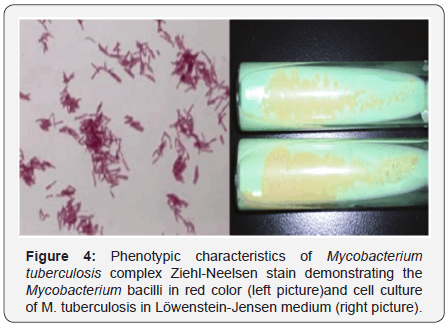
bacilli [19]. The staining use in mycobacteria identification is the
Ziehl-Neelsen staining [22]. This technique is based in the usage
of carbol-fuchsin dye, with a posterior wash with acid alcohol
and ending with methylene blue dye [23]. However, since M.
tuberculosis has a high concentration of fatty acids in its cellular
wall, acid alcohol is insufficient to completely remove the carbolfuchsin;
therefore, the bacteria are visualized as red-pink with a
contrasting blue background. Then, M. tuberculosis is known as
an acid alcohol fast bacilli (AAFB) [24].
The genome of M. tuberculosis has a high guano sine and
cytosine concentrations [20] with around 65.5%. From the
approximate 4,000 genes in its genome, more than 200 are
destined to fatty acid metabolism [18]. This could be related with
the growth capacity in the tissues of the host, where fatty acids
may represent the main carbon source for the bacteria [18].
Tuberculosis bacilli are usually acquired by the inhalation of
droplets, which are expelled by sick subjects [25], but also by
ingesting contaminated non-pasteurized milk [11] and by direct
inoculation [2]. Among the droplets that come with cough, the
biggest fall to the ground and those of 1-5μm diameter [26],
which can be considered small, remain suspended in the air and
can evade filtering processes in the upper respiratory airway
to be quickly deposited in the pulmonary alveoli [14]. These
secretions, known as “Flügge droplets”, contain the tuberculosis
bacillus. Flügge droplets have been associated with the
transmission of the disease from a previously infected patient that coughs, speaks and sneezes close to a healthy subject,
because of the high probability of inhalation and deposit of such
droplets into the alveoli [27,28].
Wells and Riley expanded this concept through an equation
(Wells-Riley equation). They evaluated the risk of transmission
based on the amount of Flügge droplets required to infect, which
they called “quanta”, inside a defined space [27]. Furthermore,
Wells demonstrated that after the evaporation of the fluid of
such droplets, the nuclei can still mobilize in an aerodynamic
fashion for their quick dispersion in the environment [28].
The development of the disease results from the
evolutionary strategy of M. tuberculosis [6]. The initial innate
immune response progresses to an adaptive form due to the
immunological activation, which tries to control the infection.
Only the equilibrium of the effector mechanisms of the cytokines
regulates the response and determines whether the host is
susceptible or resistant to infection by M. tuberculosis [29].
Although the initial immune recognition could be generated
by dendritic cells or even neutrophils [14], the first human
cell that encounters the bacterium is the alveolar macrophage,
which phagocytes it but does not destroy it since this process is
inhibited by the pathogen itself [8].
It is suggested that the endosome containing the bacterium
does not maturate because M. tuberculosis interrupts its
acidification by the release of ammonia [30] given that the
mycobacteria prefer to grow in a neutral pH [31]. Additionally, the
phagosome-lysosome fusion event is stopped by mycobacteria,
which inhibited the Ca2+ sensor synaptotagmin (Syt-7). Finally,
diverse phenolic glycolipids of the cellular wall of M. tuberculosis
prevent the production of pro-inflammatory cytokines in
macrophages [32].
There is a potent Th1-mediated immunity in macrophages
that produce nitric oxygen to try to kill the bacilli by competing
with the oxygen at the binding site on the cytochrome c-oxidase
in the cellular respiration chain of the mycobacteria. Regardless,
Mycobacterium is capable to arrest its replication down
regulating its respiratory pathway. Then, it avoids the collapse of
the electron flow by the parallel up-regulation of a less efficient
Cytochrome bd Oxidase (Cyd) and of the Nitrate Transporter
Nar K. They can restore the redox balance by working as an
antioxidant (Cyd) and a machinery to reduce nitrates (Nar K)
inside the bacteria cell, reducing ATP synthesis in the pathogen
[33].
Therefore, Mycobacterium has two progressive metabolic
states according to the increase in the pressure by hypoxia: one
of non-replicating persistence with oxygen levels reduced to 1%
saturation and the other of a complete shutdown of metabolism
when oxygen is consumed at less than 0.06% saturation [34].
Thus, the bacterium can safely live inside the macrophage under
both aerobic and anaerobic conditions, where it is capable of
replicating every 25 hours [35].
Apoptotic macrophages have an important role in adaptive
immunity because the dendritic cells acquire exogenous antigens
from apoptotic vesicles of infected macrophages [36]. The
dendritic cells transport M. tuberculosis to the lymphatic system,
migrating to the regional lymph nodes where they present the
mycobacteria antigens to T-lymphocytes [14].
The previously referred response in which macrophages
experience apoptosis in order to present antigens to dendritic
cells is evident in M. tuberculosis strains with low virulence
power. Indeed, it has been reported that macrophages infected
with the non-virulent strain H37Ra produce prostanoids,
including PGE2, that generate apoptosis and a Th1 immune
response. However, if the macrophages are infected with the
virulent strain H37Rv, they preferentially synthesize lipoxin
A4, a negative regulator of the acute inflammatory response,
that promotes necrosis in macrophages instead of apoptosis
[37]. Highly virulent M. tuberculosis strains stimulate necrosis
by mitochondrial transition of permeability, which allows the
release of cytochrome C and the further activation of the caspase
pathway [38]. Together, these changes generate accelerated
cell death with plasmatic disintegration that protects the
mycobacteria [37]. On the other hand, virulent strains of M.
tuberculosis in pre-necrotic macrophages continue duplicating
and are propagated when the cell is lysed [39].
A competent immune response is evident when the alveolar
macrophage and the dendritic cell present the microbial antigen
to CD4+ T-lymphocytes. Both IL-6 and IL-12 are then required
to generate and expand Th1 CD4 clones for the production
of cytokines, as INF-γ, that stimulate the differentiation of
circulating monocytes to macrophages. In turn, the macrophages
phagocyte the bacilli [40-42] and promote the production
of nitric oxide syntheses 2 to control bacterial growth [43].
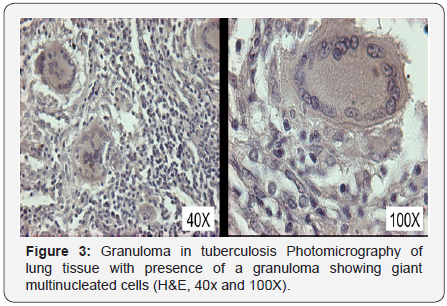
Furthermore, the stimulation of Th1 immune response is
associated with the formation of granulomas at the infection site
for the encapsulation of the bacteria inside of the macrophage
(Figure 3). The granuloma is composed by a central nucleus of
caseous necrosis, surrounded by neutrophils, dendritic cells,
natural killer cells, epithelioid cells (macrophages that haveundergo a maturation process), macrophages originated from
circulating monocytes, and multinucleated gigantic cells known
as “Langhans giant cells” that derive from epithelioid cells,
delimited by T- and B-lymphocytes, and fibrosis [44].
The specific recruitment of macrophages to the site of
infection is related with TNF-α secreted by infected T-lymphocytes
and macrophages, which secures high levels of the chemokines
CCL2/MCP-1, CCL12 and CCL13, all of which bind to the CCR2
receptor for the enrolment of macrophages [45]. Certainly,
TNF-α is recognized as the orchestrator of granuloma formation
and maintenance since its presence is mandatory for the correct
traffic of macrophages, and because mice deficient in TNF-α lack
the ability to form organized granulomas [46].
Although the granuloma was previously regarded as a
protective strategy of the host, now they are recognized to
have a role as the niche for M. tuberculosis replication [6].
Particularly in the case of mycobacteria expressing the ESX-1
secretion system encoded by the RD1 locus, also recognized as
highly virulent bacteria, it has been suggested that granuloma
might support bacilli proliferation since RD1-deficient bacteria
induce arriving macrophages to the infection site with slower
movement and without morphological characteristics similar to
those presented by leukocytes undergoing chemo taxis, which
are features of motile macrophages that cover more area for
phagocytosis [46]. Consequently, RD1-mediated chemo tactic
motility of macrophages is exploited as an advantage by M.
tuberculosis, since infiltrating macrophages phagocytose the
contents of dying cells and therefore the number of infected cells
can increase [46].
This statement is supported by the fact that RD1-mutant
bacilli induce slower kinetics of macrophage recruitment
and function, which is related with inhibition in bacterial
proliferation [46]. On the other hand, the mycobacteria that
survive the immune response latently stay in the granuloma
for decades and generate tuberculosis infection, asymptomatic
and non-transmissible [11], with the potential of reactivation
[29]. The patients with latent infection are considered as noninfective,
despite having a 10% risk of showing reactivation
through their life time [47].
There must be a balance of both pro-inflammatory
and anti-inflammatory responses to limit mycobacterial
proliferation within the granuloma, and a dysregulation leads
to reactivation and disease. With the reactivation, there is a
localized inflammatory response of high magnitude: the center
of the granuloma curses with necrosis and then undergoes
liquefaction, which provides a rich source of pathogenic
elements [6]. In fact, it has been identified that once the central
necrosis process has started, macrophage-differentiated foam
cells, which are characterized by high lipid accumulation,
appear flanking the necrotic site to accumulate caseous residues
and function as hosts for the nutrition of mycobacteria by the
supply of lipid bodies [48]. A virulent bacillus that is releasedfrom the granuloma then can migrate to other sections of the
lungs to induce more lesions, and if the granuloma breaks into
the airway, the mycobacteria is able to be transmitted to other
subjects [45].
Most of the infected patients with M. tuberculosis are
asymptomatic [6]. M. tuberculosis has the potential of infecting
every organ in the body, and pulmonary tuberculosis is the most
frequent infectious presentation because of the cavitations that
harbor a high number of bacteria with the potential of spreading
with coughing. In contrast, the extra pulmonary presentation of
tuberculosis may be contagious [8], depending on its localization.
It is considered that the patient has primary tuberculosis if he
develops the disease as a response to the initial exposition to
the bacilli; while, secondary tuberculosis relies on a reactivation
[49]. However, the Center for Disease Control (CDC) of the United
States describes the presentations as follows: latent infection,
tuberculous disease, and extra pulmonary tuberculosis. In this
section we will address each of them.
It is estimated that close to 2 billion people around the
world are latently infected with tuberculosis bacilli [50]. After
the inhalation of M. tuberculosis, the immune system of the host
restricts the permanence of the bacilli without replication or
with a low rate of replication inside the alveolar macrophage.
This explains the asymptomatic clinical curse of patients who
are non-infectious after typically normal thoracic radiographs,
negative microbial culture, sputum tests, and an absence of
tuberculosis disease [26]. This presentation is detectable by
the tuberculin cutaneous test or by the IFN-γ release assay, but
2-8 weeks after the infection are required to allow the immune
system to react against tuberculin and detect the infection [26].
The viable bacilli can persist in the necrotic material of the
granuloma through several years; however, under an immune
suppression state as the co-infection with the HIV virus, the
presentation advances to active disease [51].
This kind of tuberculosis occurs when the bacilli evade the
immune system and multiply enough to progress from the latent
disease [26]. The tuberculous disease is related with several
conditions, including poverty, alcoholism, immune suppression,
and advanced age [52]. It is estimated that without treatment,
around 5% of the patients evolve within the firsts two years
after the infection to the tuberculous disease and 5% develop it
at some point of their lifetime [26].
The most common presentation is the pulmonary
tuberculosis, and unlike the latent form, tuberculous disease is
contagious. Obtaining a diagnosis is possible after radiological
tests, and/or with the identification of bacteria in either sputum
or culture and recognizing bacterial colonies in agarose [26]. The
patient commonly curses with productive cough, mucopurulentsputum, weight loss, progressive adynamia, anorexia, fever,
nocturnal diaphoresis, general discomfort and sometimes
hemoptysis of variable volume [52].
The disease is insidious at the beginning, with initial nonproductive
cough that turns productive with purulent sputum
and hemoptysis, associated with pulmonary tissue destruction
by cavitations and pleuritic pain secondary to the inflammation
in the pulmonary parenchyma [51]. Additionally, patients
can develop paratracheal lymphadenopathy because of the
dispersion of the bacteria from the lungs to the lymphatic
system. They curse with pleural effusion if the bacilli infiltrate
the pleural space because of the rise in the size of the primary
lesion [51]. If the size of the effusion is small, then it may be
spontaneously solved or increase its size together with fever,
pleuritic pain, and dyspnea [51].
With the progression of the disease, the clinical manifestations
increase in intensity. Emaciation commonly occurs secondary
to anorexia and metabolic alteration by inflammation [51].
Acropachy is a late sign of poor oxygenation and is present in
chronic patients [51]. The extended disease generates dyspnea
or orthopnea because the pulmonary diffusion capacity is
diminished with the increase of the interstitial volume [51].
The study of the sputum, the culture in liquid mediumor
in agar in low-income countries because of the low costs,
susceptibility to antibiotics, and recently, the use of rapid tests
as Interferon-Gamma Release Assays (IGRAs), are recommended
as standard methods for the diagnosis of tuberculoses disease
[53]. Besides, it is recommended to run tests in at least two
specimens of morning sputum in order to search for the bacilli
when the patients show persistent cough for three or more
weeks without response to treatment, particularly if they live
in underdeveloped countries from Africa, Asia, and Europe [52].
Hematic biometry studies can demonstrate anemia, the cause
of asthenia and adynamia, and leukocytosis in response to the
infection [51].
Furthermore, in recent years a clear correlation between
the reactivation of previously dormant tuberculosis bacilli and
immunosuppressive therapies against autoimmune diseases has
been identified. The first example comes with lupus, in which
patients undergoing high cumulative dose of steroid or other
immunosuppressive therapy are most susceptible to tuberculous
infection by M. tuberculosis. In this case, it is common to find extra
pulmonary involvement and dissemination through the body by
the modulation of IFN-γ production and the proliferation of cells
reacting to mycobacterial antigens [54]. A delayed diagnosis
is common since extra pulmonary tuberculosis can mimic
inflammatory arthritis, systemic lupus erythematosus pleurisy,
and even cellulitis [55].
On the other hand, it is widely known that the reactivation
of latently infected subjects with the tuberculosis bacilli is
possible if the patients are treated with biological agentslike TNF-α antagonists, as the chimeric monoclonal antibody
Infliximab, since the firsts months of treatment [56,57]. This
drug is part of the treatment scheme given to rheumatoid
arthritis patients to diminish pathologic inflammatory events.
As with lupus, rheumatoid patients are more susceptible to
develop extra pulmonary presentation of tuberculosis. Indeed,
some studies have estimated the relative risk of reactivation
of tuberculosis driven by Infliximab increases up to 25 times
[58]. This effect can be explained by the pharmaco dynamic
effect of Infliximab on the formation of complexes with both the
monomeric and trimeric forms of soluble and trans membrane
TNF and in consequence, on the inhibition of the phagocytosis of
mycobacteria by macrophages [59]. Together with a reduction in
IFN-γ levels by immune modulators, the process of mycobacteria
killing is compromised [60].
This presentation occurs in sites other than lungs, and
affects 10-43% of the infected patients, depending on the
race, age, presence of a previous disease, M. tuberculosis strain
genotype, and immunological state [51]. The probability of extra
pulmonary tuberculosis is augmented in immune suppressed
subjects, particularly in those infected by HIV. In this situation,
patients may present both tuberculous disease and extra
pulmonary tuberculosis [26]. Extra pulmonary tuberculosis
patients are recognized as highly contagious as in the case
of subjects with presentations in the oral cavity or the larynx
and those who possess open abscesses with the bacilli in high
concentrations [26]. Otherwise, patients with extra pulmonary
tuberculosis are not infective because the bacilli are contained
inside the tissues.
In this section we will analyze the most lethal presentations
of extra pulmonary tuberculosis, known as miliary tuberculosis,
meningeal tuberculosis and tuberculous lymphadenitis.
Disseminated tuberculosis or miliary tuberculosis is a severe
form of extra pulmonary tuberculosis in the dispersed infection in
blood. The word “miliary” alludes to the radiographic appearance
of millet seeds widely distributed across the pulmonary field
[26]. This condition is more common in children of under 5
years of age, alcoholic subjects, those with chronic renal failure
or malnourished, and immune suppressed patients with HIV coinfection,
diabetes, under treatment with corticosteroids, and/
or undergoing chemotherapy [26,61]. Its presentation depends
on the massively phohematic dissemination from pulmonary or
extra pulmonary focus and the embolization in vascular beds
from different organs in the body [61].
Miliary tuberculosis has been related with an insufficient
immune response by the host against M. tuberculosis. Since the
innate and the adaptive immunity systems act coordinately
in a synergic way, an incomplete control of the bacterium is
identified in both and allows the proliferation of the bacteriumand its systemic dissemination. In the innate immune system,
the strong bind M. tuberculosis-macrophage has been identified.
This process is mediated by mannose-binding lectins, surfactant
protein A, and the complement protein C1q, whose mutations
are risk factors for the bacterium uptake and the posterior
development of tuberculosis [62]. Additionally, if mutations in
Toll-like receptors (TLR)-2 and/or 4 are present, the intracellular
recognition of the bacilli would be compromised [62]. On the
other hand, the adaptive immune system action is related with
T-lymphocyte function, but the presence of HLA-Bw15 and HLADRB1*
15/16 haplo types in the host is related with tolerance to
the bacterium and the further presentation of the disease [63].
The miliary form of tuberculosis is multi organic, progresses
quickly and can be difficult to diagnose because of its unspecific
symptom atology, which includes chronic fever with mourning
peaks, nocturnal diaphoresis, weight loss, anorexia, nonproductive
cough, and asthenia [51,63]. Highly irrigated organs,
as spleen, liver, bone marrow, kidneys, and adrenal glands, are
frequently affected [61]. Besides, most of the patients with
miliary tuberculosis also have pulmonary tuberculosis and
around 25% of patients with miliary tuberculosis also have
meningeal damage [26].
The pathognomonic sign of miliary tuberculosis is the
presence of granulomas in the affected organs together with
small grey/red uniformly round macular lesions [61]. The
diagnosis of this presentation of tuberculosis requires the
presence of miliary infiltrate by thoracic radiography or by
computed tomography, although the diagnosis can also be done
identifying miliary tubercles by laparoscopy or surgery in the
involved organs [61]. However, reports have shown that miliary
tuberculosis can be misdiagnosed as another infectious disease
and only correctly diagnosed until necropsy [51].
Despite being commonly present with low bacterial counts,
the most dangerous localization of tuberculosis is the central
nervous system (CNS) [64]. Nearly 1% of all clinical cases of
tuberculosis affect the CNS, being malnourished children and
patients infected with HIV the principal risk groups [65]. Around
30-40% of the affected patients die [66] and more than 50%
of the survivors remain disabled [67]. The CNS infection with
the bacilli causes tuberculomas in the brain surface, commonly
referred to as Rich focus or meningitis [68]. They constitute the
most important pathway for the introduction of the bacteria into
the subarachnoid space [65]. This presentation occurs when the
mycobacterium is hosted in the tissue surrounding either the
brain or the spinal cord, with the concomitant immune response
towards both the bacterium and its released antigens [26]. This
immune reaction may block the cerebrospinal liquid flux in the
host to generate hydrocephaly, ischemia, and infarcts, mostly in
the anterior basal ganglia [68]. Indeed, it has been suggested that
the metalloproteinase (MMP)-9, produced by microglial cellsto control the infection, could in fact degrade the extracellular
matrix of the brain [67]. The symptom atology-headache, altered
mental status that progresses to coma, neurological signs
including monoplegia or paraplegia, and cervical rigidity after
exposition to tuberculosis or in high risk groups-indicates the
necessity to consider this disease in the differential diagnosis
[26,51,69]. Other signs of neurological involvement include
motor deficit as well as optic atrophy or damage in other cranial
nerves [68].
This presentation is noticeable with hydrocephaly,
meningeal reinforcement by imaging procedures due to
arachnoiditis, infarcts secondary to vasculitis and particularly,
tuberculomas, which are the chief finding in these subjects [64].
Tuberculomas are identified as multiple ring-shaped lesions
of diverse sizes, usually found together with symptom atology
of focal neurological damage without evidence of systemic
disease [70]. However, their presence in frontal, temporal, and
optochiasmatic regions of the brain, is associated with a bad
prognosis [68]. It has been reported that nearly half of the
tuberculomas are recognized during the treatment of patients
[64]. This effect may be related with the tuberculosis-immune
reconstitution inflammatory syndrome (IRIS) seen in HIVinfected
individuals treated with the HAART scheme as soon as
two weeks after starting HIV therapy [66]. In such patients, a
great number of neutrophil infiltrates at the CNS are associated
with inflammasome activation and the concomitant abundance
of myeloperoxidase, cathepsin G, lipocalin-2, MMP-8 and MMP-
9, among other pro-inflammatory cytokines [66]. Magnetic
resonance is the diagnosis test of election, mostly when it is not
possible to work with biopsies [68]. Nevertheless, the clinician
should be aware that several reports of tuberculomas mistakenly
diagnosed as oncological diseases can be found and that the
successful diagnosis of tuberculosis in the CNS remains a clinical
challenge [70].
Tuberculous lymphadenitis is the most common extra
pulmonary tuberculosis presentation [71] due to the immune
cell migration following tuberculosis infection [72], particularly
in pediatric population and in women between 30-40 years of
age [73]. Several risk factors are related with this presentation,
including a Southern Asian ethnicity, previous oropharyngeal
expositions to the M. tuberculosis complex, hormonal influences,
immune suppression, and effects related to the immunization
with the BCG vaccine [73,74]. The patient typically shows a
slowly progressive and painless swelling of a unilateral group
of lymph nodes, mostly affecting the peripheral and cervical
lymph nodes, which can grow up to 8-10cm in diameter during a
period of 1-2 months [72,73]. Besides, since lymph nodes are the
primary site for adaptive immunity generation, the pathological
study of the affected lymph nodes usually demonstrates the
presence of granulomas, more frequently found in subjects coinfected
with HIV [75]. Besides, the concomitant presences of pulmonary tuberculosis, as well as of disseminated diseases and
of systemic symptoms including fever, are more common in HIVpositive
patients than in HIV-negative individuals [73].
Finally, it is also necessary to mention the pediatric
tuberculosis presentation. It is estimated that close to 11%
of all the affected patients are under 15 years of age [76], a
number that can grow to 20% in regions with high incidence
of tuberculosis [77]. The fast and imperceptible progression
towards the tuberculous disease is a particular characteristic of
the pediatric patient [78]. Just as in adults, the most common site
of tuberculosis in children is the lung [51]. The most reported
symptoms are chronic cough (longer than 4 weeks of duration),
dyspnea, asthenia, thoracic pain, hemoptysis, deviation from
the expected trajectory in the growth curve, and bad response
to the initial treatment. All of them variate according to the age
and the immunological state of the minor [51,76]. Infants and
scholar children show highly variable presentations that can
be clinically and radio logically similar to common respiratory
infections; however, during adolescence the manifestations are
the same as in adults [51]. Although normal chest radiographies
in children are found-even with confirmed tuberculosis, the
presence of pronounced hilaradenopathy and with or without
compression of the airway, the diagnosis of pulmonary
tuberculosis is suggested [76]. Less than 15% of the cases are
positive for the identification of M. tuberculosis in sputum while
growing the bacteria in culture has a rate of success around
30-50% [78]. Given the difficulty of obtaining sputum samples
from children, it has been reported that the combination of
chronic cough, weight loss and anorexia, is highly predictive of
tuberculosis in patients under 15 years of age [51]. The habitual
absence of sputum production with cough in children is related
with a lower contagiousness [26].
The detection of M. tuberculosis infection is still a challenge.
Despite the most widely employed diagnostic techniques are the
cutaneous tuberculin test and the IFN-γ release test (described
later in this section), the isolation of the bacilli is not possible
when the infection has been eradicated by the immune system
[79]. Furthermore, distinguishing between latent infection and
active tuberculosis is still complicated using these diagnostic
approaches [2]. Below we briefly describe the most commonly
employed diagnostic tests for the diagnosis of M. tuberculosis
infection.
The detection by bacilloscopy and the bacteria culture are
the definitive diagnosis of active tuberculosis and the primary
examination methods for patients with tuberculosis symptoms
[80]. The diagnosis is based on the identification of the bacilli
in sputum. But, when the sputum cannot be obtained, it is
induced or the sample is taken from nasopharyngeal aspiration
[81]. The staining techniques employed are Ziehl-Neelsenfor conventional microscopy and auramine-rhodamin for
fluorescence microscopy [80].
In the particular case of the central nervous system,
the identification of the bacteria by Ziehl-Neelsen has been
complicated because of the required volume of cerebrospinal
liquid. In consequence, a modified Ziehl-Neelsen stain based
on centrifugation on slides using cerebrospinal liquid samples
has been recently demonstrated to be more sensitive than the
conventional stain for diagnosis in CNS and provides a diagnosis
with 0.5ml samples [82]. The WHO guidelines establish that
two-three slides per patient must be analyzed in successive
days; the results should show evidence of the bacilli in more
than two days for a definitive diagnosis of the infection [83].
However, the employment of direct microscopy as an option for
rapid detection is difficult in the clinical practice because only
44% of new cases in adults and 15-20% in children have the
bacilli in sputum samples [80]. Because of that, it is advisable
to perform other diagnosis methods (including tuberculin test,
IFN-γ release test, PCR, and/or chest radiography) when there is
suspicion of infection, even with a negative isolation result [80].
Cellular culture is the reference method to detect M.
tuberculosis with the employment of Middle brook 7H11
and Lowenstein-Jensen media. Although cell culture is more
sensitive than detecting the bacilli in sputum samples, these
two media require more than two weeks to detect colonies in
solid culture [11] (Figure 4). There currently exist multiple
commercial culture systems that are based on a liquid medium,
which require less time for the detection of bacterial growth
[80].
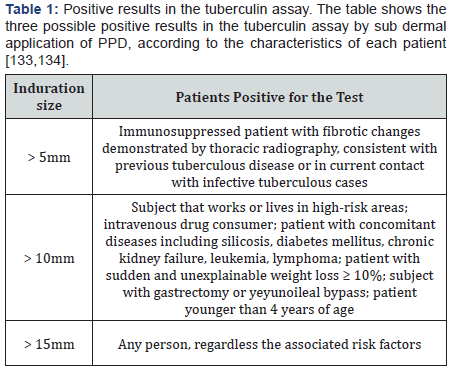
Tuberculin detects delayed cutaneous hypersensitivity,
useful for the differentiation of latent infection by M. tuberculosis
[84]. The tuberculin reaction is measured as in duration in
millimeters 48-72 hours after the sub dermal application of
purified protein derivate (PPD) of the mycobacteria under the
skin in the anterior side of the forearm [80,85] (Table 1). Both
sensitivity and specificity rely on the size of the in durationelected as cut-off value, the immune system of the patient, and
unspecific reactions, which can be due to previous exposition
to environmental mycobacteria or BCG vaccination [2]. Indeed,
clinical reports support the presence of positive PPD tests
in patients affected with cervical lymphadenitis due to non
tuberculoses mycobacteria such as the complex M. aviumintracellular,
M. haemophilum, M. simiae and M. scrofulaceum, in
whom a PPD in duration of >15mm is commonly seen [86].
Furthermore, the age of vaccination has been demonstrated
as a critical determinant of the response to PPD test. One metaanalysis
of 24 studies revealed that such vaccination during
infancy is associated with 8.5 false-positive tuberculin tests
per 100 vaccines, being 2.6 of these false positives reactions of
>15mm [87]. Since this response could be a clinical problem in
populations with high prevalence of non-tuberculous infection
and low prevalence of tuberculosis infections, previous studies
have focused on the description of common proteins among
mycobacteria that can potentially act for further Cutaneous tests.
They have shown that the L7/L12 proteins in the ribosomal
50 subunit, as well as the hypothetical proteins WAG31 and
Rv3075c, are potential candidates of common proteins in
mycobacteria [88].
This diagnostic method is based on the principle that
T-lymphocytes release IFN-γ when they re-encounter specific
antigens belonging to M. tuberculosis. To perform the test, a fresh
blood sample with a viable content of leucocytes is required. The
sample is incubated with controls and a mixture of synthetic
peptides representing the M. tuberculosis antigens ESAT-6, CFP-
10 and TB7-7 [80,89]. The IFN-γ released is then identified in
the supernatant after incubation with antigens. Alternatively,
the IFN-γ-producer cells are counted in an enzyme-linked
immune sorbent assay (ELISA) [2]. This test is useful to detect
latent tuberculosis and is more specific than the tuberculin test
because the antigens employed for the detection of IFN-γ areunique in M. tuberculosis and are not associated with either BCG vaccine or any other non-tuberculosis mycobacteria, including
M. avium [90].
This test is rapid, highly specific (>90% for pulmonary
tuberculosis) and can predict antibiotic resistance. It is possible
to amplify and detect rRNA or DNA from the mycobacteria with
the employment of either blood, cerebrospinal fluid, sputum,
bone marrow, or a tissue sample [80]. This is a useful and cheap
test for the detection of the bacilli in a diverse range of samples.
It provides bacteriological information with multiple weeks of
anticipation compared with cellular culture [91].
The radiological diagnosis of pulmonary tuberculosis is a
challenge, mainly in immune suppressed patients that present
atypical patterns [92]. Primary tuberculosis is manifested as four
entities by radiography: parenchymal disease, lymphadenopathy,
pleural effusion, and miliary disease, which can be combined in
the same patient. Parenchymal damage is identified as a dense
consolidation, predominantly in the superior pulmonary lobe
due to the reduced lymphatic drainage and the reduced local
oxygenation [93]. While, a multi-lobar disease can be identified
in around 25% of the cases [94] (Figure5). The appearance of
parenchymal damage tends to be similar to that of bacterial
pneumonia, but it can be differentiated by the presence of
lymphadenopathy and the absence of response to conventional
antibiotics [95].
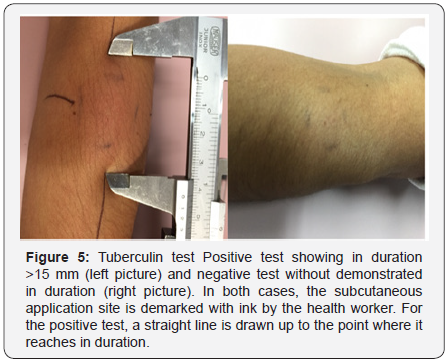
Nearly 66% of the cases are solved without sequels during the
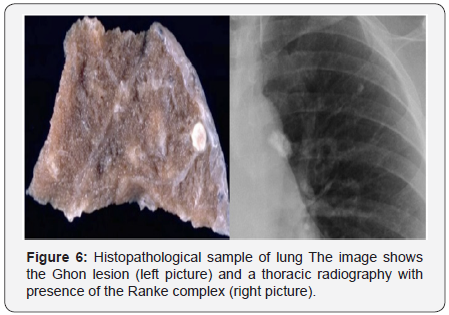
firsts two years; in the rest, the presence of acalcified parenchyma
scar, known as Ghon nodule, is commonly the only evidence
of previous tuberculosis [94,96] (Figure 6). Right unilateral
lymphadenopathy, with both ipsilateral hilar and paratracheal
involvement, is also commonly identified. The presence of
calcified hilar nodules together with a Ghon lesionis known as
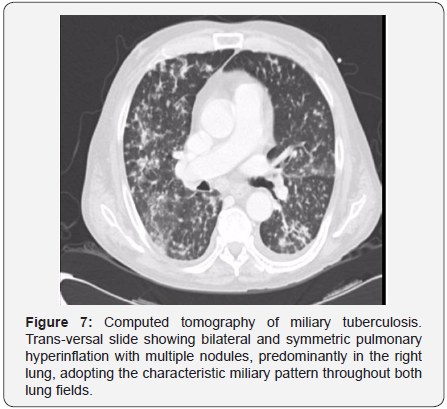
the Ranke complex [95]. Miliary disease is more common among children, elderly population, and immunosuppressed patients.
At the beginning of the clinical presentation it is associated only
with pulmonary hyperinflation [95]. However, as the disease
progresses, diffused nodules of 2-3mm diameter are identified.
They are predominantly distributed in the inferior lobules of the
lungs, may coalesce and do not calcify [95] (Figure 7).
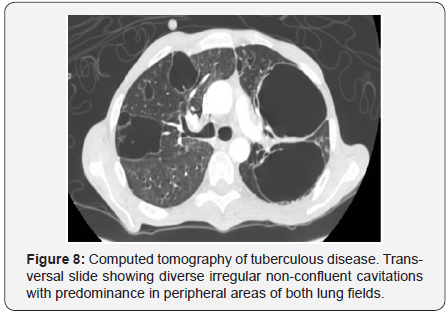
On the other hand, secondary tuberculosis is identified
focally or in patch heterogeneous consolidations found in apical
and posterior segments of superior lobules and in superior
segments of inferior lobules [94]. Cavitations, which indicate
primary disease, can progress to endobronchial areas and form
the typical “sprout tree” distribution, considered an active
tuberculosis marker. The elected method that demonstrates
an early bronchogenic spread is computed tomography [94]
(Figure 8).
The position-emission tomography with 18-fluoro-2-deoxi-
D-glucose with computed tomography (18F-FDG PET-CT) is a
non-invasive tool in which the pulmonary and extra pulmonary
variants of tuberculosis are simultaneously evaluated [92]
(Figure 9). It is not a habitual procedure for the diagnosis of
tuberculosis since it is commonly performed in the search for
cancer. Its employment is based on the detection of an increase
in the glucose metabolism, which is related with the rise of
macrophage and neutrophil activity in tuberculosis [92]. The
glucose uptake rate is reported as the standardized uptake value
(SUV), being maximal in active tuberculosis [96].
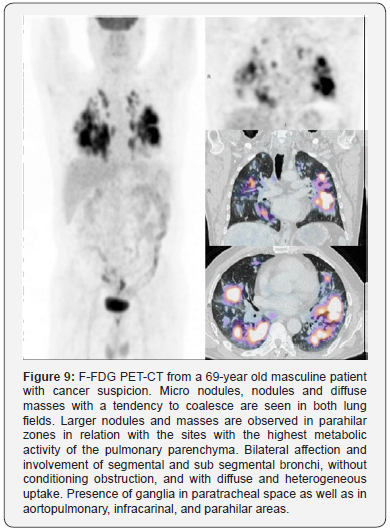
This technique is useful to evaluate the response to treatment
when the radiological features are manifested with little or null
changes [92]. There are two described patrons by 18F-FDG PET
CT: one pulmonary, with consolidations in the superior lobule and
cavitations or cavities surrounded by micro nodules and one
lymphatic, which is represented by calcifications of the lymphatic
nodules and requires the comparison of both the size and
symmetry of them, which together, indicate the compromised
nodules [97]. A clear correlation between the reductions
in the accumulation of pulmonary 18F-FDG and a successful
antibiotic treatment has been demonstrated [98]. However, the
main limitations of 18F-FDG PET-CT are its expensiveness, low
specificity, and lack in the capacity to discern a granulomatous
disease with malignant involvement [92].
In the absence of other diagnostic methods, it is possible to
employ the serum diagnostic to search for lipoarabinomannan
(LAM) with ELISA. However, the anti-LAM antibodies do
not distinguish between pathogenic and non-pathogenic
mycobacteria strains and can generate crossed reactivity against
other LAM-producer genus bacteria, such as Nocardia [99].
The presence of M. tuberculosis can be detected using a
real time PCR-based technique with both pulmonary and nonpulmonary
samples. This technique can also simultaneously find
resistance to rifampicin [100]. For this test, mutations (present
in 95-98% of all the rifampicin-resistant strains) in the resistantdeterminant
region of the rpoB gene are detected with the
employment of five specific molecular probes designed for this
purpose [101]. This is a simple, sensible and specific approach
[100]; it has proven its affectivity in isolated cases in New York,
Madrid, India, and Mexico [102]. Furthermore, since most of the
isolated mycobacteria that are resistant to rifampicin are also
resistant to isoniazid, this method could be useful to identify
multi-resistant strains [102]. Currently, the Cepheid Genexpert
system processes human samples by real-time PCR to identify
multi-resistant strains in less than two hours with a sensibility
close to that of the M. tuberculosis cell culture [102]. Besides,
since it requires a processing time of less than five minutes per
human subject, the diagnostic human error rate is considerably
reduced [101].
One of the newest techniques for tuberculosis diagnosis
is the mycobacterial DNA aptamer detection. DNA aptamers,
oligonucleotide chains that fold in specific structures to bind
to several molecules, are analog to antibodies and possess high
stability [99]. Aptamers are highly sensible and specific for the
identification of almost any molecule. Since they can be produced
by chemistry synthesis or by PCR, their cost is low, between 10
to 50 times less than the required to produce antibodies [103].
Nowadays there are DNA aptamers specific for the identification
of the polyphosphate kinase 2 (PPK2) of M. tuberculosis [103].
Researchers have recently described a diagnostic method based
on the generation of particular aptamers for the glycolipidantigen ManLAM of the Beijing strain of M. tuberculosis, which
has shown similar detection rates as the obtained with diagnostic
kits [99].
The Millennial Development Goals were eight objectives
that the United Nations Organization wanted to achieve by the
year of 2015; they included the fight against major diseases,
as tuberculosis [104]. A big effort promoting an accessible
treatment has been made, but the co-infection of M. tuberculosis
with HIV and the existence of tuberculosis strains with different
degrees of resistance to antibiotics have generated a complex
control [105]. Therefore, members of the WHO are working
on the Sustainable Development Goals (SDGs) to end with the
global tuberculosis epidemic between 2015 and 2030. With the
WHO End Tuberculosis Strategy and using a pharmacological
approach, they aim to reduce the tuberculosis-associated
deaths in 90% and the tuberculosis incidence rate in 80% by
2030, compared with 2015 [1]. In this section, we will address
the current treatments for tuberculosis and prevention with
vaccination.
Although the successful treatment rates have been
maintained since the year 2007, the emergence of M. tuberculosis
strains with extended spectrum of antibiotics resistance has
complicated the therapeutic approach to tuberculosis [104].
In fact, tuberculosis can be curable in 85% of cases if treated
correctly [80]. Even though studies have demonstrated that by
the second week of treatment the infectivity potential of the
patient is diminished, it is highly recommended to complete the
treatment scheme [2].
The first-line treatment is based on use of isoniazid,
rifampicin, Pyrazinamide, and Ethambutol [106], with a
standardized approach of both rifampicin and isoniazid for six
months and Pyrazinamide and ethambutol during the firsts
two months [77]. On the other hand, the second-line treatment
includes cycloserine, terizidone, ethionamide, prothionamide,
capreomycin, amino salicylic acid, amino glycosides such as
kanamycin and amikacin, and fluoroquinolones including
ofloxacin, levofloxacin, gatifloxacin and moxifloxacin [77].
Together with the specific treatment, the usage of adjuvant
corticosteroids has been suggested to reduce the inflammation
state of the patient [6].
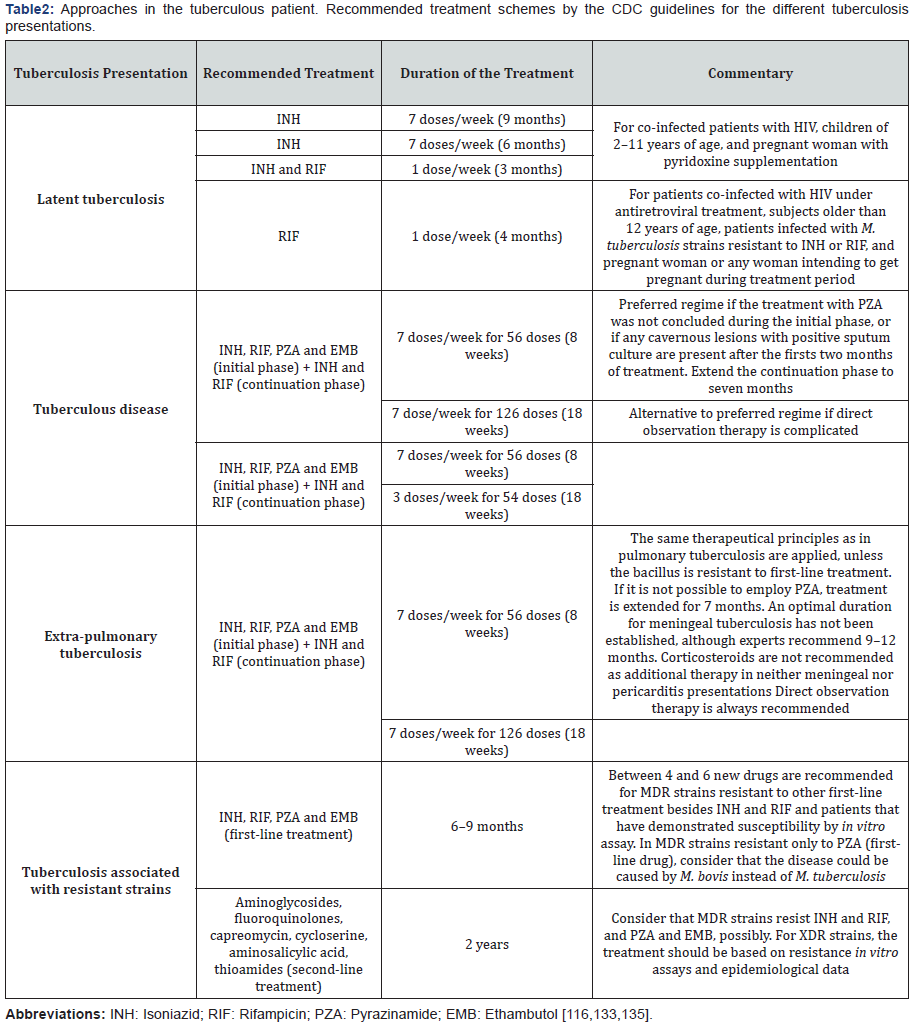
The treatment scheme depends on the immune state of the
patient and the resistance the bacterium shows; in consequence,
the pharmacological therapy must be maintained up to 24
months in some cases [6] (Table 2). A particular case is that
of patients with latent infection, for whom the recommended
treatment is isoniazid during a period of 6-9 months in order
to reduce the risk of tuberculosis disease, especially if they had
contact with patients that demonstrate the active form [77].
Isoniazid is a prodrug that requires activation by the catalase
peroxidase (KatG) to target the NADH-dependent enoyl ACP
reductase InhA, which then stops the mycolic acid synthesis
[107]. Rifampicin is a DNA-dependent RNA polymerase inhibitor
that forms a stable complex with this enzyme to prevent the
initiation of bacterial RNA synthesis [108]. Pyrazinamide is a
prodrug activated by acidic pH and transformed by the bacterial
pyrazinamidase into its active form, pyrazinoic acid, to affect
the membrane transport in the bacteria [109]. Ethambutol is
a drug that alters the structure of the mycobacterial cellular
wall inhibiting the transference of arabinogalactan into the
wall and [D-14C]-glucose into the D-arabinose residue of the
arabinogalactan molecule [110].
The preventive therapy with antibiotics or prophylaxis
in countries with high prevalence of the infection should be
limited to co-infected patients with HIV and children under 5
years of age that have contact with infected subjects at home.
Contrastingly, in countries with low prevalence, the application
of the preventive therapy is recommended in immigrants from
countries with high prevalence and people that have a close
contact with latently infected patients [6]. Furthermore, another
group that must be considered for the preventive therapy is that
of healthcare workers, in whom the conversion surveillance by
PPD can indicate the necessity of prophylaxis. Indeed, due to
their close contact with patients and/or with potentially infected
material from patients, healthcare workers are in continuous
risk of exposition and possible acquisition of the bacillus. In the
United States, the CDC recommends to adequately follow the
hospital infection control standards as a primary strategy of
prevention amongst this group of workers, since other people
inside the hospital, including patients and visitors, may not
vaccinated against M. tuberculosis [111]. In countries with high
prevalence of tuberculosis, both the surveillance and the control
among healthcare workers should be attended with specific
programs of prevention and control.
It must be considered that a preventive approach does
not only directly affect the health of the population, mostly in
underdeveloped countries, but also has a deep impact on the
economy. In the case of tuberculosis, a standard therapy of 6
months costs around $2000 dollars/patient in industrialized
countries, a cost which can be expanded up to 25 times if the
subject is infected with a multi-drug resistant strain (MDR) or
with an extensively resistant strain (XDR) [112].
Although drug resistance in tuberculosis treatment was
discovered in 1946 after the introduction of streptomycin
[113,114]. MDR strains emerged around the world more than 25
years ago, with the subsequent development of XDR and recently,
strains that resist both first and second line drugs, known as
extremely multidrug resistant strains (XXDR) [113,115]. These
groups of resistant mycobacteria are transmitted in the same way
as M. tuberculosis strains that are susceptible to pharmacological
treatment [26]. Tuberculosis by MDR is generated by M.
tuberculosis strains that resist at least the two most effective
drugs against tuberculosis, isoniazid and rifampicin, while XDR
strains also resist any fluoroquinolone and one of the three
injectable treatments (capreomycin, kanamycin or amikacin)
[115]. These bacteria can acquire their resistance through any
of two ways, known as primary and secondary resistances. The
primary form occurs in patients initially exposed and infected
by resistant organisms, and the secondary develops during the
treatment of tuberculosis, either due to an inaccurate regime
(the patient did not follow the prescribed scheme properly) or
mal absorption of the drugs [116].
Even though the exact proportion of MDR and XDR strains is
not known, in 2008 the WHO estimated that 3.6% of the incidentcases in the world were caused by MDR; half of them were
identifiable in India and China. This Organization also reported
that MDR strains were responsible for approximately 150,000
deaths during the same year [115] since less than 1% of MDR
bacilli cases were treated properly [77].
In countries such as Estonia, Lithuania, Azerbaijan and
Ukraine, XDR strains are associated with more than 10% of all
the MDR cases. In Eastern Europe and in the Sub-Saharan Africa
the HIV/AIDS epidemic has promoted the prevalence of MDR
strains [115]. On the other hand, we now know that more than
12% of the incident cases and more than 50% of the previously
treated cases in countries that used to belong to the Union of
Soviet Socialist Republics are MDR strains [115]. It has been
reported that is the case of the city of Minsk, in Belarus, more
than one third of the incident tuberculosis cases were caused
by MDR bacteria [113], which reveals that the approach to MDR
tuberculosis has been insufficient. This information, along with
the fact that routine cellular cultures and drug susceptibility
assays are done in only 22% of the countries around the world,
worsens the prognosis for patients [115]. Furthermore, use of
combined treatments of first and second line drugs to treatment
of MDR and XDR strains is recommended, according to the results
from the antibiotics susceptibility assay. Longer treatments
are also suggested, even though it is known that the approach
tends to be less effective and badly tolerated by the patient.
Because of that, it is estimated that the percentage of patients
with tuberculosis due to MDR strains heal in less than 69% of
the cases, even after treatments lasting 18 months. According
to experts, an ideal drug combination for a treatment includes
at least three active drugs against MDR and XDR strains, which
can have a complementary, synergic and potent effect against
diverse subpopulations of M. tuberculosis [77].
Thanks to the employment of next generation sequencing
technologies, the genome of M. tuberculosis has been
demonstrated to be highly dynamic [117]. In fact, the main
reason of resistance is a spontaneous genetic mutation in
the bacteria, and not horizontal transference [115,118].
Nevertheless, this is a debatable statement since some reports
have demonstrated a lack of association between treatment and
the presence of mutations in the mycobacteria [119]; however,
others show that recent mutations generating hetero resistance
(presence of sensitive and resistant allele in the same sputum
sample) are associated with induction of treatment [120]. In
fact, it is known that in the presence of active tuberculosis,
subpopulations of resistant mycobacteria emerge and can be
the dominant strain under the pressure of the treatment [115].
Isoniazid resistance is related with mutations in its activator,
katG, as well as with the gene mabA [121]. On the other hand,
rifampicin resistance is almost always associated with a punctual
mutation in the generpoB, which codifies for the β subunit of
RNA polymerase [12]. These mutations that confer resistance
happen at predictable rates: for isoniazid, a resistance rate of
10-6 is expected; while a resistance close to 10-8 is expected forrifampicin [115]. Besides, a patient may present pulmonary
tuberculosis by MDR strains that respond to treatment in
different ways, depending on the anatomical localization of the
pulmonary lesion [122].
Therefore, multiple efforts have been made in research to
improve the treatment scheme for the benefit of the patient.
There currently are at least ten different compounds in clinical
development for tuberculosis approach, most of which are new
drugs while others follow the strategy of therapeutic positioning
[77].
The BCG vaccine consists in a live and attenuated strain of M.
bovis, a species related to M. tuberculosis, derived from a virulent
strain after 231 passages in potato and bills medium [85,123]; it
is currently the only vaccine available against tuberculosis. This
vaccine primarily protects against the most aggressive forms of
the disease (miliary and meningeal) in children [15]. It has been
employed since the early 1920s [124] when clinical studies done
between 1921 and 1927 in France and Belgium demonstrated
its high efficacy to protect against tuberculosis in children [85].
Initially it was administered orally, although by the 1930s the
intradermic administration began in Scandinavian countries
[85]. After the Second World War, the vaccine was given to all
European children and the WHO extended its employment in
endemic areas outside Europe by the beginning of the 1950s
[85]. In 1974, the BCG vaccine was introduced in the extended
immunization program of the WHO [125]. Nowadays, routine
BCG vaccination in children is encouraged by the WHO and most
of the countries around the world use it [126]. Today, more than
3 billion people have been inoculated with BCG, making it the
most employed vaccine around the world [85].
The WHO currently recommends that all infants born in
countries with a high risk of infection by M. tuberculosis must
be vaccinated with an intradermic dose of BCG just after birth
[85]. Despite its wide distribution, randomized controlled
trials have revealed that BCG vaccine presents high clinical
variability in the conferred protection against pulmonary
tuberculosis, with an efficacy in the range of 0-80% and an
average of 50% [123]. Experts have proposed that this variation
may be caused by nutritional and genetic characteristics of the
population, as well as by previous exposition to non-tuberculous
mycobacteria, differences in natural history of infection and
disease, methodological variances between the trials, and even
differences between the BCG formulations and in the time of
their applications [126,127]. One systematic review of all the
reported BCG trials until 2014 aimed to analyze the efficacy of
BCG vaccines using the univariate meta-regression method. The
study revealed that the protective effect of BCG for pulmonary
tuberculosis is greater if it is applied to subjects younger than
school age and at latitudes farthest from the equator, with an
absent or low protection in trials between latitudes 0º-20º and
20º-40º, compared with those at >40o [5]. Presently, multiplelaboratories have produced their own BCG strains. Among the
BCG strains currently in use, there can be listed the Tokyo,
Glaxo/Denmark, Moreau and Pasteur strains [123].
The BCG vaccine is accessible, stable, and secure; it is not
affected by maternal antibodies and generates long-term
immunity with only one dose [123]. The lowest rate of protection
has been identified in countries with the highest incidence of
tuberculosis, where results with the BCG vaccine have suggested
a prevention success of deaths associated with tuberculosis close
to 5% [85]. Therefore, there are two postulated strategies to
improve the vaccination approach: to replace or to potentiate the
BCG [128]. Replacing the BCG involves the employment of a more
potent vaccine obtainable via genetic mutants of M. tuberculosis
or the manipulation of the BCG vaccine to create recombinant
strains (rBCG) that secrete antigens or other cytokines [128].
Work has been done in rBCGs that express the Ag85B antigen
of M. tuberculosis, early secreted in the infection by this bacillus
[129], to produce vaccines as the rBCG30 as well as strains that
amplify the T CD8+ response, expressing listeriolysin to perforate
the phagosome membrane (rBCG ΔureC:Hly) [128]. Additionally,
researchers have generated rBCGs that promote a Th1 immune
response via the production of cytokines including IFN-γ, IL-2,
IL-2, and granulocyte and macrophage colony stimulating factor
(GM-CSF) [123].
Regarding the second strategy, the design of vaccine boosts
that increase the immune response generated by the vaccination
in the childhood, as adjuvants, is postulated [128]. Studies have
proposed several adjuvants including IC31, GLA-SE, AS01, and
CAF01 as well as cationic liposomes that can promote a cellular
immune response against M. tuberculosis [128,130-135].
Despite the efforts made by diverse organizations and
several governments to reduce the incident cases of infection
by M. tuberculosis, the disease remains a global public health
problem. The diagnostic and therapeutic tools we currently
have are insufficient for a correct approach in the patient. This
limitation has stimulated the emergence of strains with diverse
ranges of resistance to current treatments, a fact that worsens
the prognosis of infected subjects. Furthermore, the rise in the
number of patients affected by HIV/AIDS increases the risk of
propagation of the bacillus and induces a shorter survival time
of HIV/AIDS patients. Therefore, there must bea continuous
research that allows us to identify new treatment schemes
useful against resistant strains. The development of accessible,
fast, sensitive, and specific diagnostic methods should also
be promoted by clinical microbiology laboratories of the
governments around the world. Their efforts should reveal the
presence of Mycobacterium strains either sensitive or resistant
to standard treatments in order to start a timely approach in
the patient. Finally, we must promote the prevention of the
acquisition of the bacillus by improving the BCG vaccine and
its administration in highly vulnerable areas. Altogether, these efforts may promote a better prognosis for the population and
should reduce the incident cases of tuberculosis infections.
To know more about Open Access International
Journal of Pulmonary & Respiratory Sciences please click on: https://juniperpublishers.com/ijoprs/index.php

Comments
Post a Comment