Tuberculosis and Cerebrovascular Disease: A Review on Pathogenic Mechanisms-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS INTERNATIONAL JOURNAL OF PULMONARY & RESPIRATORY SCIENCES
Abstract
The burden of tuberculosis and Cerebrovascular
diseases is enormous world-wide. Changing global trends have
increasingly made high, middle and low income countries face one of
these less exposed burdens. Evidences linking tuberculosis with
Cerebrovascular disease have supported the notion of both intraluminal
and extraluminal pathology. Vessel wall invasion and inflammation that
happens with Tuberculousmeningitis (TBM) has a high level of evidence
supporting Tuberculosis as the cause of vasculitis. The basal exudates
of TBM have natural course involving the smaller vessels and larger
vessels in early and later stages respectively. SPECT along with MR
imaging of brain parenchyma and vessels increases the sensitivity and
specificity of diagnosis. Steroids play a vital role in reducing the
frequency of stroke occurrence and mortality in TBM patients when
initiated in early stages. Autoimmune vasculitis secondary to systemic
tuberculosis has been demonstrated in Eale’s disease affecting the
retinal vasculature and Takayasu arteritis affecting the Aorta or its
branches. Chronic inflammation in systemic tuberculosis stimulates
atherogenesis/plaque rupture in the Tunica intima leading to increased
cerebrovascular events. But the level of evidence to this statement is
low and therefore warrants further investigation.
Abbreviations: ANCA: Anti Neutrophil Cytoplasmic Antibody; ATT: Anti Tubercular Therapy; CD: Cluster Determinant; CSF: Cerebrospinal Fluid; CT: Computerised Tomography; ESR: Erythrocyte Sedimentation Rate; HIV: Human Immunodeficiency Virus; HLA: Human Leucocyte Antigen; Hsp: Heat Shock Protein; IL: Interleukin; IFN: Interferon; MPB: Mycobacterial Protein Fraction from BCG; MRI: Magnetic Resonance Imaging; PCR: Polymerised Chain Reaction; PET: Positron Emission Tomography; SPECT : Single Photon Emission Computerised Tomography; TA: Takayasu Arteritis; TB: Tuberculosis; TBM: Tuberculous Meningitis; Th: T helper cell; TNF: Tumour Necrosis Factor; VEGF: Vascular Endothelial Growth Factor; WHO: World Health Organisation
Introduction
Tuberculosis is a major health hazard to humanity for
centuries and is one of the oldest known infections affecting the
mankind. In 2015, WHO reported an estimated 10.4 million new cases of
Tuberculosis. An estimated 1.4 million HIV negative TB cases died in the
same year making the Case fatality rate 17%. 6 countries of the world
namely China, India, Indonesia, Nigeria, South Africa and Pakistan had
60% of the cases [1]. Unfortunately, the above counties do not lag
behind the western world with regards to the burden faced due to stroke.
Yearly 15 million people suffer from stoke globally, a third of whom
die and another one third left with permanent disability. A larger
population below 40 years suffered from stroke in India compared to the
Western world.
The association of Tuberculosis with stroke is a
major issue in the developing countries where the incidence of TB cases
is high. Also the recent issues of immigration have made the western
world face this dreaded disease. Tuberculous meningitis (TBM) is a known
extra pulmonary site for TB infection which is associated
with high incidence (20% [2] -30% [3]) of stroke. But does stroke result
only from TB meningitis? Does systemic TB infection pose a risk for
stroke? This review explores the various mechanisms for stroke
occurrence in patients infected with Tuberculosis.
Classification of Vascular Involvement in Tuberculosis
Vascular involvement culminating in stroke in patients infected with tuberculosis occurs due to the following mechanisms
- Direct invasion of vessel wall
- Immunological reaction against the vessel wall
- Inflammatory mechanisms promoting atherogenesis/plaque rupture
Among these mechanisms, level of evidence is high for
direct vessel wall invasion. Postulated models are available for other
mechanisms.
Direct Invasion of Vessel Wall
Tuberculous meningitis (TBM) is the prototype entity
which results in stroke due to vessel wall infiltration. Meningial
deposit of the bacilli follows emboli from tuberculous lesions in
lung, kidney and bone. Children are more affected than adults.
A dense gelatinous fibrinocellular exudate accumulates in the
leptomeninges, particularly in the interpeduncular fossa. The
exudate widens two layers of leptomeninges and then spreads
anteriorly to encircle anterior cerebral vessels and optic chiasma
and laterally into the sylvian fissure to encircle the carotid,
middle cerebral vessels and the penetrating branches. Posterior
spread marks the pontomesencephalic and medullary cistern
involvement with blocking of Foramen of Lushka [4].
Pathogenesis
The cellular reaction in the meninges is directly proportional
to the duration of the illness. A chronic course witnesses
lymphocytic and plasma cell infiltration. Following the cellular
reaction inflammatory vasculitisoccurs. This is marked by 3
stages.
- Infiltration
- Proliferation
- Necrotizing changes
The tubercle bacilli encountered by astroglia, endothelial cell
and monocyte induce cell adhesion molecule expression and thus
neutrophil recruitment throughdiapedesisinto the CSF. Neutrophil
secretes vasoactive peptides leading to disruption of blood brain
barrier and recruitment of lymphocytes and macrophages through
cytokines. Fibrinousexudates containing these inflammatory cells
accumulate in the basal meninges. The lymphocytes along with
plasma cell infiltrate the vessel wall progressively from Tunica
adventitia towards Tunica intima (Figure 1). Among the cytokines,
CSF IL-6 and TNF – alfa have positive correlation with severity and
progression of TBM. The infiltration of the intima is followed 2-3
weeks later by proliferation of smooth cells and collagen. This
with or without thrombus formation causes vessel wall occlusion
and results in infarction (Figure 2). Among the less common
mechanisms which result in stroke are the fibrinoid necrosis of
vessel wall andvenous sinus thrombosis, implicated in the rare
occurrence of hemorrhagic lesion in TBM. Thrombosis in TBM is a
rare phenomenon and is due to hypercoagulable state in the acute
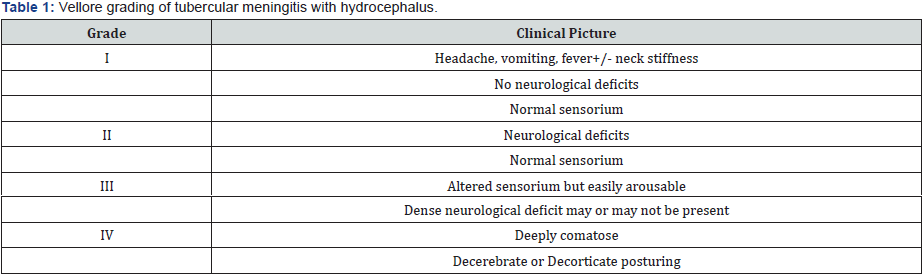
phase of more severe forms (Stage 3 and 4) of TBM (Table 1) [5].

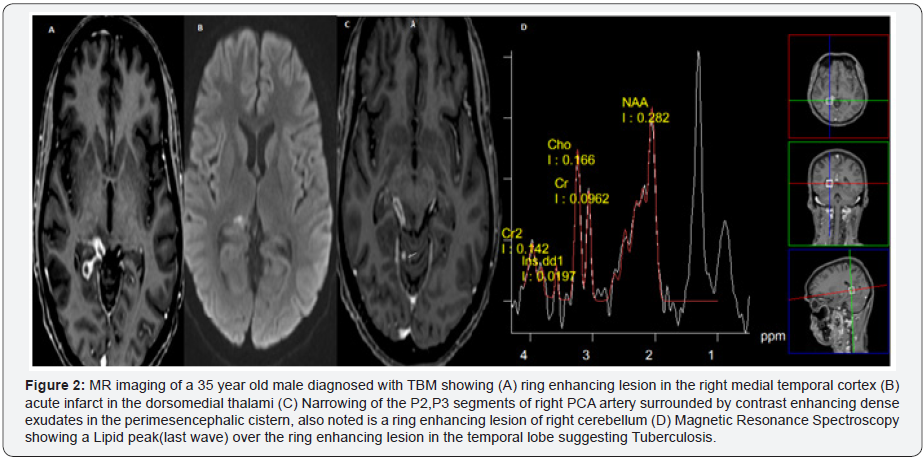
Clinical features
Patients present with weakness in monoplegic pattern in
the acute stage reflecting the small vessel occlusion and then in
hemiplegic/quadreplegicpattern, decortication posture in the
chronic stages reflecting middle cerebral artery and internal
carotid arteryocclusion, thus inferring that longer duration
of TBM is associated with higher incidence of stroke. TBM
vasculitis is insidious and is not characterized by Transient
Ischemic Attacks associated with atherothrombotic strokes.
Also aphasia, agnosia and hemianopia are less uncommon.
Cranial neuropathies occur along with motor deficit due to direct
compressive effect from the exudates and when present along
with infarction increases the possibility of TBM rather than acute
bacterial meningitis. Detecting the focal neurological deficits
is clinically challenging owing to the altered sensorium in TBM.
Stroke in TBM reflects a poor outcome both in terms of morbidity
and mortality. Advanced stage of tuberculous meningitis, basal
exudates, optochiasmaticarachnoiditis and vision impairment are
significant predictors of stroke in patients with TBM [3].
Neuroimaging
MRI is sensitive than CT in detecting the infarcts in patients
with focal neurological defects and also in patients with altered
sensorium with a high suspicion of vascular pathology. TBM
vasculitis affects specific vessels and specific areas of brain.
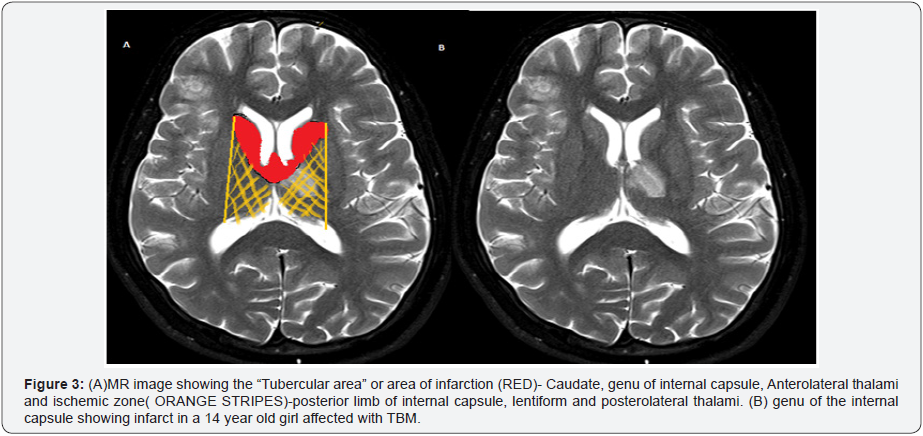
Basal ganglionic infarctions comprising the head of the caudate,
anteromedial thalami, anterior limb and genu of internal capsule
are the commonest affected regions of the brain and are commonly
referred to as the “Tubercular Zone” (Figure 3). Small vessels
namely medial striate, thalamotuberal and thalamoperforate
arteries are implicated.
The classical TBM triad in cerebral angiogram includes the
1) narrowing of supraclinoid portion of internal carotid artery
2) widely sweeping pericallosal artery or outward bowing of
thalamostriatevein, and 3) delayed circulation in middle cerebral
artery with scantly collaterals and early draining veins suggesting
a dilated ventricular system and constricted carotid and middle
cerebral vessels due to arteritis. Such a triad cannot be appreciated
in all the patients and a larger population (57%) showed normal
angiographic studies.
Collaterals arise around the arteries affected by TBM and have
been classified into three forms.
- net like clusters of thin vessels in the region of basal ganglia and base of the brain which resemble Moyamoya like pattern
- transdural external–internal carotid anastomosis
- intracranial cortical anastomosis with altered architectural arrangement
SPECT scan is more sensitive in picking up the hypoperfusion
than angiographic studies. Adding MR imaging helps to differentiate
a vasculitic cause from a periventricular edema secondary to a
hydrocephalus when the SPECT shows hypoperfusion in the basal
ganglia region.
Treatment
Patients benefit with combined steroid and standard
antituberculartherapy (ATT). Beneficial effects of steroid
especially intravenous dexamethasone have been demonstrated
in various studies. Steroids reduce the frequency of infarction
and mortality when started early (Stage 1 TBM), but they do not
improve the long term disability of the TBM patients affected with
hemiplegia prior to starting medications. Also, the mortality rates
in later stages of TBM are not affected by steroids.
Immunological Reaction Against Vessel Wall
Tuberculous infection anywhere in the body can mount
an immunological reaction against the vessel wall due to the
molecular mimicry between TB bacilli and vessel wall antigens.
There are two autoimmune vasculitic conditions in which TB has
been strongly proposed as the trigger factor
- EALE’S DISEASE
- TAKAYASU ARTERITIS
Eale’s Disease
Eale’s disease is an obliterative vasculopathy involving the
retinal vessels in young males (>97%) predominantly between
20-30 years of age.
Etiopathogenesis: Multiple models have been proposed
linking the inflammatory and autoimmune mechanisms in
Eale’s disease. Of these the most favoured is the one which links
Eale’s disease with Tuberculosis. More than 70% of patients
had a positive Mycobacterium species detected by PCR from
the epiretinal membrane. Also MPB 64 gene and genome of
Mycobacterium tuberculosis were demonstrated in more than
50% of patients from the epiretinal membrane and vitreous
humour respectively. Individuals with predisposition to HLAB5,
DR1 and DR5 are more vulnerable. Elevated serum IL-6, VEGF
levels and increased monocytes are seen. Non-classical CD 16 +
monocytes with a high expression of Toll like Receptor 2 on the
cell surface are observed confirming cell mediated autoimmunity
directed against the vessels. The above mechanism leads to
inflammation of the peripheral retinal vessels with occlusion and
retinal infarcts occur along with neovascularization and vitreous
hemorrhage.
Clinical Features: Patients remain asymptomatic early in
the course of the disease. They notice floaters and blurring of
vision which might worsen to a level of complete blindness. Visual
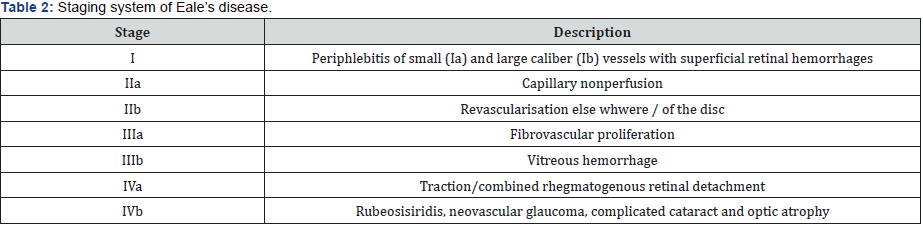
symptoms are bilateral in 50%-90% population. A new staging
system based on the ocular findings has been proposed (Table
2). Nervous system abnormalities including myelopathy, ischemic
stroke, bilateral white matter abnormalities in MR imaging and
vestibuloauditory dysfunction have been reported less commonly.
Treatment: Corticosteroids form the mainstay of treatment.
Patients not responding to steroids are switched to anti VEGF
monoclonal antibody Bevacizumab. Patients showing strong
positive reaction to purified protein derivative are also given
a 9 month ATT. Studies support Vitamins and antioxidants.
Ophthalmological interventions like photocoagulation and
vitrectomy are indicated in proliferative stage and vitreous
hemorrhage respectively.
Takyasu Arteritis
Takayasuarteritis (TA) is a systemic inflammatory
granulomatous vasculitis of large and medium vessels affecting
the aorta and its branches.
Etipathogenesis: Tuberculosis has been strongly linked
to TA in various studies. One study demonstrated IS6110 and
Hup B gene sequences of Mycobacterium tuberculosis in more
than 70% of aortic tissues of TA patients. Also Real time – PCR
was 93.87% and 98.69% sensitive and specific in detected the
M. tuberculosis bacilli from the aortic tissues of TA patients. An
Indian study estimates a 44.8 times higher chance of developing
TA in patients with active tuberculosis compared to healthy
population [6,7]. Latent tuberculosis detected by Tuberculin
Skin Test and Interferon Gamma release assays is more positive
in TA patients than general population. A molecular mimicry
occurs between the Heat shock protein (hsp) expressed by
humans and M. tuberculosis bacilli. Due to an unknown trigger
human expresses the mhsp 65 protein of M. tuberculosis in the
aorta. This induces the Histocompatibility complex class I chain
A (MIC-A) on vessel wall recognised by the Natural killer cells of
innate immunity. These cells release perforin on the vessel wall
and induce inflammation by recruiting Th1 and Th 17 Cells. These
cells along with their corresponding cytokines induce aortic
arteritis [8].
Clinical Features: The commonest presentations are
hypertension secondary to renal arterial stenosis and cardiac
failure. Neurological involvement consists of headache (50-
70%), visual disturbances (16-35%) and strokes (5-9%) due to
involvement of carotid and vertebral arteries. Diagnosis confirmed
using both blood tests and imaging. Raised ESR, positive ANCA
titres are sensitive tests. MR/CT angiogram showing a focal/
segmental non arteriosclerotic narrowing or occlusion of aorta,
its primary branches or large arteries of extremities increases the
specificity. Vascular PET is the gold standard investigation when
available.
Treatment: Corticosteroids are the mainstay in acute stage
followed by immunomodulation with either cytotoxic agents like
cyclophosphamide, azathioprines, methotrexate or biologicals
like TNF inhibitor-etanercept.
Inflammatory Mechanisms Promoting Atherogenesis/ Plaque Rupture
Commonest cause of ischemic stroke globally is
atherothrombosis and thrombo embolism. Systemic tuberculosis
as a risk factor promoting atherogenesis in the cardiovascular
and cerebrovascular vessels is an issue of debate. Studies have
both argued and refuted this hypothesis and therefore the level
of evidence is low. A Taiwan based 3 year follow up study using
a large cohort showed that patients affected by tuberculosis and
completed treatment had a 1.52 times higher risk of developing
a stroke than age matched population, after adjusting for other
stroke risk factors [9]. Another Taiwan study refuted the increase
occurrence of stroke in non-central nervous system Tuberculosis
[10,11]. Extrapolating the information from the studies supporting
higher cardiovascular events related with systemic tuberculosis,
to the cerebrovascular events gives us the mechanism underlying
the atherogenesis.
Pathogenesis
- Increased expression of pro-inflammatory cytokines (i.e., IL-1, IL-2, IL-6, IFN-γ, TNF-α)
- Monocyte/macrophage immune activation
- CD4+ TH1 and TH17 cell immune activation
- Auto-immunity mediated by antibodies against mycobacterial HSP65( similar to Takayasu arteritis)
Together these mechanisms cause endothelial activation,
increased expression of adhesion molecules in the tunica intima.
This attracts the immune cells (lymphocytes, monocytes) and
thereby promotes atherosclerosis by formation of foam cells in the
sub intimal space. This increases the plaque size in the subintimal
space and then recruits the smooth cells to form the cap of the
plaque along with collagen. Inflammation also causes rupture of
the plaque as a result of Matrix metalloproteinase.
The current evidence is insufficient to relate the atherogenic
cerebrovascular events to Tuberculosis and will require further
investigations.
Conclusion
Evidences linking tuberculosis to Cerebraovasular events
through systemic vasculitis and atherogenesis are surprising.
Models proposed both in the immunological and inflammatory
frontiers look explanatory, but are incomplete. Further research
is requires to investigate the link between Tuberculosis and
Cerebrovascular disease.
To know more about Open Access International
Journal of Pulmonary & Respiratory Sciences please click on: https://juniperpublishers.com/ijoprs/index.php
To know more about Open access Journals
Publishers please click on : Juniper Publishers
To know more about juniper publishers: https://juniperpublishers.business.site/

Comments
Post a Comment