Role of Ipratropium Bromide and Tiotropium in Chronic Obstructive Pulmonary Disease-Juniper publishers
JUNIPER PUBLISHERS-OPEN
ACCESS INTERNATIONAL JOURNAL OF PULMONARY & RESPIRATORY SCIENCES
Abstract
Introduction: Chronic obstructive pulmonary
disease (COPD) remains a major public health problem. COPD is costly
disease. Many patients suffering from chronic obstructive lungs diseases
are of poor economic status, mostly illiterate, therefore has a direct
bearing on patient compliance. Tiotropium is a new anticholinergic
therapy for chronic obstructive pulmonary disease that differs from
ipratropium by its functional relative selectively for musarinic
receptor subtypes and which allows once - per - day dosing. This study
presents a cost-effectiveness, efficacy, and side-effects of Ipratropium
bromide and Tiotropium in COPD.
Methods: Prospective study was conducted In
Kathmandu University Teaching Hospital, between the year 2008 and 2009
in terms of cost- effectiveness, efficacy, and side-effects of
Tiotropium and Ipratropium amongst COPD patients.
Results: Tiotropium and Ipratropium were
prescribed in total of 57 patients (30 in ipratropium bromide and 27 in
tiotropium bromide) for the management of COPD of outpatients. There
were no significant differences in age, height, weight and baseline lung
function parameters (FEV1 and PEF) between the two drugs i.e.
ipratropium bromide and tiotropium bromide. Significant improvement in
lung function parameters were found in each respective group of drugs
after bronchodilator therapy. Tiotropium results in significant
reduction of Chronic Obstructive Pulmonary disease exacerbations and
significant improvement in quality of life, lung function, and dyspnoea
compared to ipratropium. The additional cost to achieve these favorable
outcomes was cheaper than Ipratropium bromide. (Nepalese Rs. 7.03 per
day for tiotropium as compared to Rs. 9.06 for Ipratropium)
Conclusions: Tiotropium results in significant
reduction of Chronic Obstructive Pulmonary disease exacerbations and
significant improvement in quality of life, lung function and dyspnoea
compared to Ipratropium.
Introduction
Chronic obstructive pulmonary disease (COPD) remains a
major public health problem. It is the fourth leading cause of chronic
morbidity and mortality in the United States and is projected to rank
fifth in 2020 in burden of worldwide, according to a study published by
the World Bank/World Health Organization [1].
Chronic Obstructive Pulmonary Disease is characterized by chronic
airflow limitation that is not fully reversible and usually progressive
characteristic symptoms include chronic and progressive dyspnoea, cough
and sputum production, which result in significant impairment in
exercise capacity and quality of life [2]. Worsening lungs function is also associated with frequently increasing disease severity [3].
This contributes to a more rapid decline in lungs function,[4] increased mortality and further reductions in quality of life[5].
COPD is costly disease. Many patients suffering from chronic
obstructive lungs diseases are of poor economic status, mostly
illiterate, therefore, it has a direct bearing on patient compliance. In
coming year, the burden of COPD will increase as reflected by
increasing inpatient and outpatient flow. There is an acute need for
more effective treatment options to reduce the burden of this disease
for patients, care givers and society [6].
COPD is a growing problem in developing countries including Nepal.
There is a striking direct relationship between the severity of COPD and
the cost of care [7].
Cost for patient varies across countries because the cost depends on how health care is provided and paid [8].
Tiotropium is a new anticholinergic therapy for chronic obstructive
pulmonary disease that differs from ipratropium by its function
relatively selectively for musarinic receptor subtypes and which allows
once-er-day dosing. The present study is a prospective study designed to
compare efficacy, safety and cost effectiveness of tiotropium bromide
and ipratropium bromide in the treatment of COPD. The efficacies of two
drugs were measured with reference to the pulmonary function test based
on spirometry. The safeties of drugs were measured in terms of side
effects produced during treatment. The costs of drugs were calculated in
terms of cost per day of drugs used by the patient.
Methods
Prospective study was conducted in Kathmandu
University Teaching Hospital, between the year 2008 and 2009 in terms of
cost-effectiveness, efficacy, and side-effects of tiotropium and
ipratropium amongst COPD patients. The patients with COPD, visiting the
out patients department of Dhulikhel hospital, Kathmandu University
Teaching Hospital, between December 2008 to June 2009, were selected for
the study. Patients were selected from outpatient department from
Kathmandu University Teaching Hospital. Current or ex-smokers with
relatively stable COPD and a FEV1 < = 70 % of the
predicted normal were selected for the study. Patients were also
required to be aged >40 years. Patients with the history of asthma
requiring regular supplemental oxygen and patients with recent upper
respiratory tract infection or a significant disease other than COPD
were excluded. Patients were selected according to the selection
criteria. Fifty seven (57) consecutive patients who fulfilled the
inclusion criteria were further divided in tiotropium group and
ipratropium group: 27 in tiotropium group and 30 in ipratropium group.
Patients received 18 μg triotrpium rotacap once daily and other received
40 μg of ipratropium rotacap four times a day.
After taking informed consent, the patients were
enrolled for the study. Patients were followed up for 6 month. The tool
used was a set of prepared questionnaire and spirometry for each patient
whose diagnosis was based on clinical evidences of the diagnostic test
reports. All the patients were asked for information as specified in the
questionnaire. The spirometry of all the patients before and after
using of tiotropium and ipratropium were taken mainly for the
improvement in lung function test and testing the efficacy of two drugs.
Direct cost of tiotropium bromide (TIOVA-®) and ipratropium bromide
(IPRAVENT®) inhaler cost per day were calculated in Nepalese Rupees
(NRs).
The data obtained after structured questionnaire and
from medical records were compiled. The compiled data were analyzed
using SPSS-11.5 and the graphs were plotted using Microsoft Excel. Both
descriptive and inferential statistics were used to show the frequency
of occurrence and to establish the relationship between the variables.
The descriptive statistics included the percentage and frequency,
analysis of the frequency through graphs and lines. Mean, standard
deviation and t-statistics were calculated. Through these analysis
comparisons and interpretations were established.
Results
Demographic Characteristics
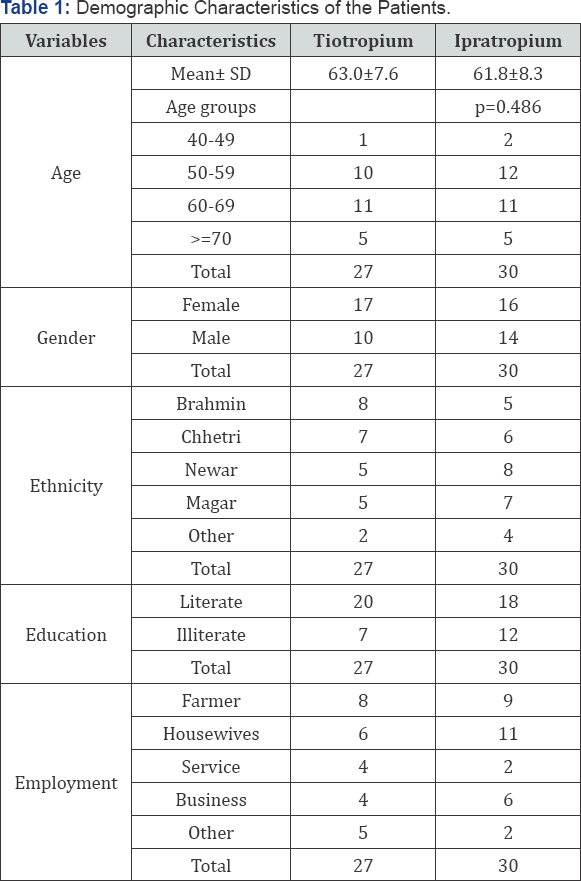
Out of 57 Patients using two different drugs,
Brahmin, Chhetri and Newar constitute equal proportion of the population
(22.8 %) while Magar (21.05 %) and other cast groups like Giri,
Pariyar, Lohala, Gurung, Sherpa and Singh constitute the least
percentage (10.5 %) of the study population as shown in Table 1.
It was found that most of the patients were literate in both of the
drug groups (66.67 %) and only one third (33.33 %) of the patients were
illiterate. The occupational characteristics showed that farmers and the
housewives constituted majority of population. Service, business and
other occupations like driver and daily wage (non-agriculture) were
recorded during the entire study.
The mean age of the COPD patients was found to be 63 ±
7.6 ranging from 49 to 78 for tiotropium bromide users and 61.8 ± 8.3
with a range of 48 to 78 for the ipratropium bromide users. The
distribution of the age in both groups seems to be similar as there is
no difference between both of the ages (p-value 0.486). Altogether,
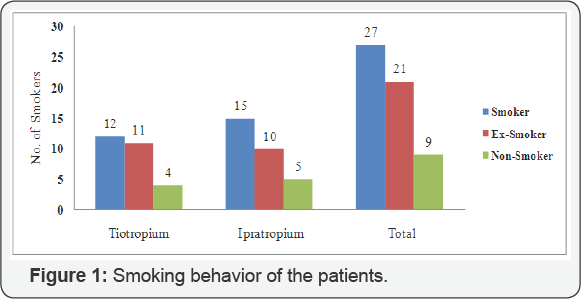
84.21 percent of the patients were smokers with 47.37 percent as
currently smoking and 36.82 as ex-smokers while 15.78 percent of the
patients were non smokers in both of the groups. Among the patients
using tiotropium bromide, 85.19 percent were smoker and only 14.81
percent as non smokers. Around the same proportion of the patients using
ipratropium bromide were smokers (83.3 %) and 16.7 were found to be non
smokers as shown in Figure 1.
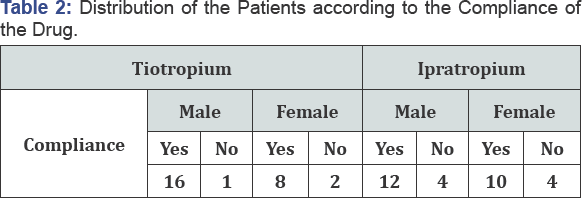
Users' behavior is also a determinant factor to the
drug effectiveness. Patients using ipratropium bromide are less regular
on taking the medicine. Out of 30 patients, only 22 patients were
regular users. Similarly, among the 27 patients consuming tiotropium
bromide, only 3 were not regular for their medicine Table 2.
The compliance of the drug is affected by various
factors. Time and frequency as well as the price are the major factors
determining the compliance. In the case of tiotropium bromide, the
possibility of disobedient on taking the drug remains less because the
drug is needed to take once a day. Patients usually do not forget to
take once. But in the case of ipratropium bromide, the case is
different. Patients need to focus their attention to take their drug for
four times a day and sometimes the regularity gets break up.
Cost is another factor underlying the compliance.
Tiotropium bromide with the brand name (TIOVA) costs 211 NRs per pack
that contains 30 capsules. Looking at first, it is expensive to purchase
but when we calculate the price, then the cost per day is only NRs 7.03
per day. The next drug, ipratropium bromide (with the drug name
(IPRAVENT) per pack can be purchased on NRs 68 that also contains 30
capsules but that is required to take four times a day. Calculating the
cost, it is only available in NRs 9.06 per day.
Among the users of tiotropium bromide, fewer side
effects were seen than among ipratropium bromide users. Urinary
retention (3), constipation (4), dryness of mouth (13), confusion(1)
were the prominent side effects seen to the users of Urinary «tension
Constipation Blurted vision Dryness of mouth Confusion Side effects as
shown in Figure 2

Ipratropium bromide has larger scale of side
effects. The prominent side effects were urinary retention (9),
constipation(11), blurred vision (1), dryness of mouth (8) and confusion
(5).
Four kinds of comparisons have been made in order to
find the differences between tiotropium and ipratropium. The lung
function (FEV1 & PEF) before and after administration (15
days) of drugs were measured in each patient. The null hypothesis in
these the cases would be that there is no difference in lung function
between these drugs before and after administration or the FEV1 and PEF
levels contains no differences. Alternative hypotheses are, then
established for the difference between the drugs.
1. FEV1 before and FEV1 after the drug administration of tiotropium bromide
2. FEV1 before and FEV1 after the drug administration of ipratropium bromide
3. PEF before and PEF after the drug administration of tiotropium bromide
4. PEF before and PEF after the drug administration of ipratropium bromide

From the above t-statistics analysis as shown in Table 3,
it seems that there are significant differences between before and
after the use of the drug in the variables tested (within the drugs) at
95 % confidence interval where the distribution follows the normality.
T-statistics shows significant differences with respective p-values. The
t-statistics of tiotropium bromide and ipratropium bromide has the
higher values in both of the cases. So they are effective drugs to use.
Similarly, the best performance of one of the drugs has been calculated
as shown below in Table 4.
From the data shown in Table 4,
their mean percentage increment in FEV1 & PEF before and drug
administration in each of drug was calculated separately. There is
significant difference between percentage increment in FEV1
(p=0.033) & PEF (p=0.024) level between tiotropium and ipratropium
after administration. So we can conclude that tiotropium has better
effect than that of ipratropium. Thus tiotropium has better lung
function than ipratropium.

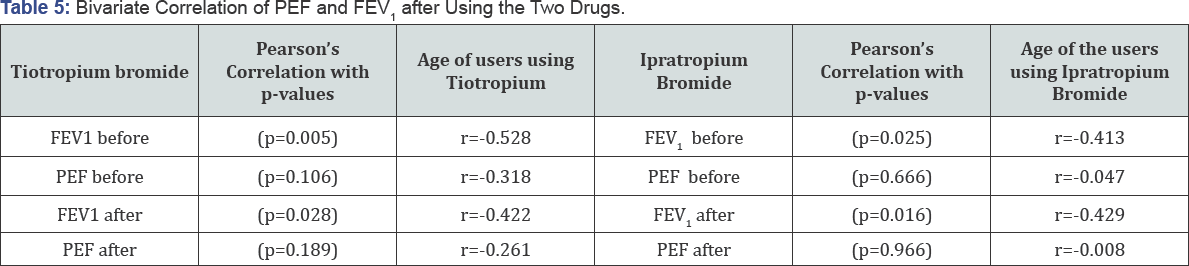
The correlation between age of the patients and lung
function test parameters (FEV1& PEF) before and after administration
of tiotropium and ipratropium were found as shown in Table 5.
In all cases, correlation coefficient (r) is found to be negative. From
this we can conclude that as the age of the patients increases their
lung function decreases.
Discussion
Tiotropium and ipratropium were prescribed in total
of 57 patients (30 in ipratropium bromide and 27 in tiotropium bromide)
via DPI (Rotacap) for the management of COPD. There were no significant
differences in age, height, weight, BMI and baseline lung function
parameters (FEV1 and PEF) between the two drugs i.e.
ipratropium bromide and tiotropium bromide. Significant improvement in
lung function parameters were found in both respective groups of drugs
after bronchodilator therapy. However comparing the improvement of the
lung function parameters between two drugs, there was a significant
difference between them (p= 0.033 for FEV1 and 0.024 for PEF respectively).
Patients using ipratropium bromide were more prone to
side effects like constipation, blurred vision, confusion and then
dryness of mouth. The side effect more prone to tiotropium was dryness
of mouth than ipratropium bromide. The utilities values were derived
independently from the efficacy data; however they did not represent the
efficacy observed in the clinical trials data [9-11]. The compliance recorded was higher in case of tiotropium bromide.
The cost of treatment with tiotropium bromide was
found to be much lesser than with of ipratropium bromide. The
Oostenbrink study compared costs in the Netherland and Canada, and found
that in the Netherland tiotropium was the least expensive treatment,
followed by salemeterol then ipratropium[12].
When cost are calculated by severity, the additional cost of toitropium
is always offset by saving made through reduces progression and
exacerbations.
Age has been negatively associated with both of the
parameters. The negative correlation coefficient between age and the
parameters in both of the groups show that with the increase in age, the
susceptibility of disease increases. From this we can conclude that as
the age of the patients increases their lung function decreases.
Conclusion
It can be concluded from this study that tiotropium
in a dose of 18 μg inhaled once a day via DPI is more effective and safe
than 40 μg ipratropium via DPI four times daily in improving lung
function over fifteen days. The safety profile of tiotropium was greater
than that of ipratropium with exception of dryness of mouth. The cost
of treatment with tiotropium is less than that of ipratropium. These
data support the use of tiotropium as a first line treatment of patients
with airflow obstruction due to COPD.
To know more about Open Access International
Journal of Pulmonary & Respiratory Sciences please click on: https://juniperpublishers.com/ijoprs/index.php
To know more about Open access Journals
Publishers please click on : Juniper Publishers
To know more about juniper publishers: https://juniperpublishers.business.site/


Comments
Post a Comment