The Smell, that Forgotten Sense-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF HEAD NECK & SPINE SURGERY
Introduction
Although human beings live in a constant and changing
bath of smells, when it comes to defining smell, as well as describing a
certain odor, the task becomes extremely difficult. The odor is
described by comparison. To define the smell I will make mine the words
of Marcel Proust, who says of him.
“Smell is the most personal, insubstantial, fragile
and persistent of the senses, able to evoke the most vivid memories from
a tiny drop of essence”.
A single particle of a certain fragrance is able to
open the appetite, announce danger, arouse sexual desire, evoke the most
remote sensations, and recall experiences and/or emotions of the
distant past. But speaking of odors, it is interesting to say that each
being has the genes that code the receptors of their smell and those
that code for the odors emanating from their body. The genetic
variations of HCM (Major his to compatibility complex, when determining
changes in the production of odorous metabolites; explain the odors
associated to specific HCM types. From the above it is inferred that
each individual has its characteristic odor. We think we smell with the
nose, but this is like saying that we hear with the earlobe. In fact,
the part of the nose that we can see from the outside serves only to
receive and channel the air that contains the odoriferous molecules. The
neurons that perceive these molecules, are in the depth of the nasal
cavity, in a portion of cells called olfactory epithelium. Perched
behind a closed curve type, on the roof of the nasal cavity, is the
olfactory epithelium, which only measures a few square centimeters. It
contains about 5 million olfactory neurons, in addition to their
supporting cells and stem cells. In fact, there are two portions of
olfactory epithelium (one on each side of the nose) that lie in a
horizontal line, just below the level of the eyes (Figure 1).
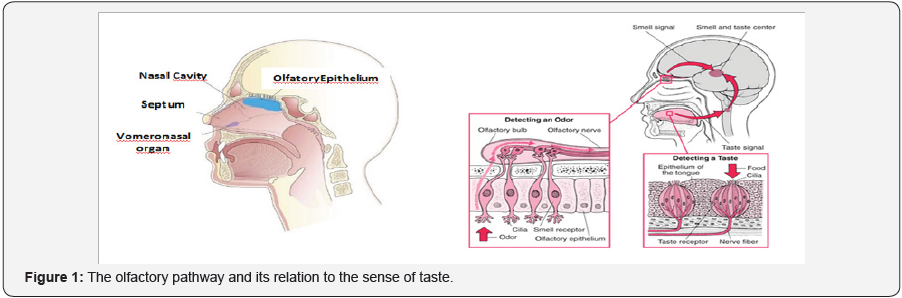
Modified from Kandel ER, et al. [1] Olfactory sensory
neurons are found within the olfactory epithelium in the dorsal
posterior fossa of the nasal cavity, as already mentioned. These neurons
project axons into the olfactory bulb of the brain, a small ovoid
structure resting on the cribriform plate of the ethmoid bone [1].
Structure of the Olfactory Epithelium
There are three types of cells: olfactory sensory
neurons, supporting cells and basal stem cells at the base of the
epithelium. Each sensory neuron has a dendrite that projects to the
epithelial surface. Many cilia protrude into the mucous layer that
covers the nasal lumen. A single axon projects from each neuron to the
olfactory bulb. Odorants bind to specific odorant receptors on the
cilia, and initiate a cascade of events leading to the generation of
action potentials in the sensory axon.
Each olfactory neuron, present in the epithelium, is
covered with at least 10 cilia projecting towards a fine mucus
bath,which is found on the cell surface. Scientists were convinced that
somewhere in these cilia, there had to be receptor proteins
that recognized and bonded to odoriferous molecules, thereby
stimulating the cell to send signals to the brain (Figure 2).
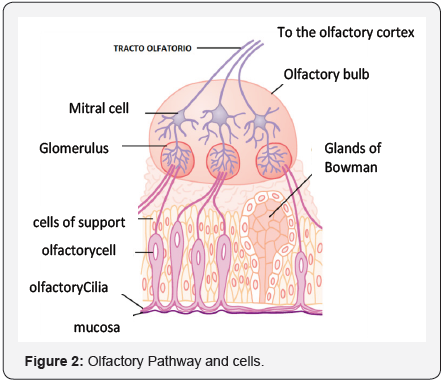
Receptor proteins would be the key to answering two basic
questions about smell, explains Richard Axel [2], an HHMI
researcher at Columbia University. The first question is how the
system responds to thousands of molecules of different shapes
and sizes, which we call odoriferous substances, “do you use
a restricted number of varied receptors or a large number of
relatively specific receptors?” And the second question, how does
the brain use these responses to distinguish between odors?
Each olfactory neuron present in the nose has a long fiber,
or axon, which is inserted through a tiny opening in the bone
above it, the cribous plate of the ethmoid, to make a connection,
or synapse, with other Neurons. This synapse forms, in fact, the
olfactory bulb, which is a part of the brain. The olfactory bulb, a
round structure like a knob, is quite large in animals with a keen
sense of smell, but decreases in relative size when this ability
decreases. Thus, although humans are more than twice the body
size of dogs, they have a much larger olfactory bulb than the
human.
Olfactory Pathway
The information is transmitted from the olfactory bulb axons
of mitral and tufted transmission neurons in the lateral olfactory
tract. Mitral cells project to five regions of the olfactory cortex:
olfactory nucleus, olfactory tubercle, pyriform cortex, and parts
of the amygdala and entorhinal cortex. Plagued cells project to
the anterior olfactory nucleus and the olfactory tubercle; the
mitral cells in the accessory olfactory bulb are only projected
into the amygdala. The conscious discrimination of the odor
depends on the neocortex (orbitofrontal and frontal crusts).
The emotional aspects of olfaction are derived from limbic
projections (amygdala and hypothalamus). In rodents and some
mammals, a well developed vomeronasal organ is related to the
perception of odors that act as pheromones; Its receptors are
projected towards the accessory olfactory bulb. The olfactory
pathway in the Rinencephalon is composed of the olfactory
nerves, the olfactory bulbs, the olfactory belts, the olfactory
striae that divide into medial, lateral, intermediate and olfactory
cortex (Figure 3).
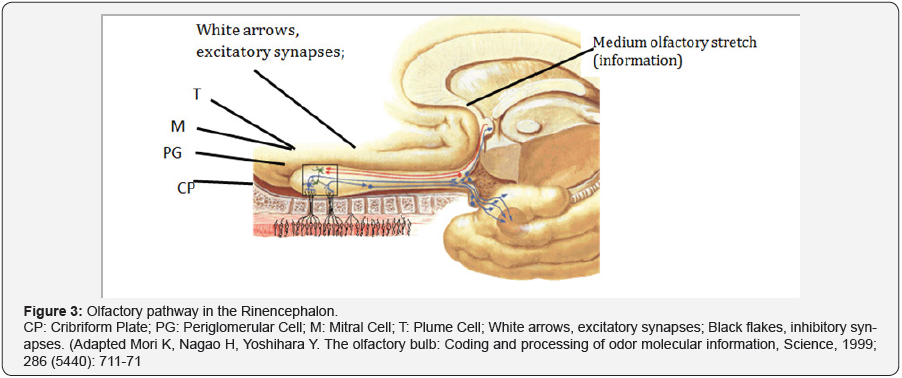
Basic Neural Circuits in the Olfactory Bulb
Olfactory receptor cells with one type of odorant receptor
project into an olfactory glomerulus (OG), and olfactory receptor
cells with another type of receptor project into a different
olfactory glomerulus. The olfactory bulb receives the olfactory
nerves and becomes thinning forming the band. The belt on
reaching the perforated space above is divided into grooves. The
lateral striae are projected towards the olfactory cortex. The
cortex consists of the piriform cortex, periamigdaloid area and
the entorhinal area. (This is the bark around the uncus). The medial striation is introduced at the anterior commissure and
communicates both bulbs. The intermediate groove is introduced
into the anterior perforated space, its function is unknown.
The rhinencephalon is in anatomical and functional relation
with the limbic system. This is the neuroanatomic basis for an
aroma to please us or to remind us of something or someone.
In general, the odors are composed of several molecules
and each of them activates several Specific receptors. Thus a
complex combinatorial code is generated which forms the socalled
“odoriferous pattern” of a substance. These patterns are
the basis of our ability to recognize and remember so many
different odors (Figure 4).
As we see for all this signal to occur the odoriferous receptors
that are proteins of the cell membrane of olfactory cilia associated
to protein G are needed. Odoriferous receptors closely resemble
rhodopsin, the receptor protein present in rod-like cells of the
eye. Rhodopsin and at least 40 other receptor proteins cross
the cell surface seven times, giving it a characteristic snake-like
shape. They also function similarly, interacting with G-proteins
to transmit the signals into the cell. Because many receptors of
this type share certain DNA sequences, Buck designed probes
that would recognize these sequences in a group of rat DNA. He
found the genes in 1991 [3].
First, they observed that the RNA obtained from rat olfactory
epithelium contained a large family of genes that could range
from 70 to 200. When comparing these gene products with the
gene libraries (Genebank) they were found to correspond to
(olfactory) genes. The second step was to sequence the proteins
of some of these genes. As a result they obtained a series of
proteins with 7 transmembrane domains similar to other
receptors already known as the adrenergic and cholinergic
receptors. They observed that in these proteins there was a great
variability in the presence of amino acids, especially in domains
3, 4 and 5, which could explain an enormous variability of gene
control. They then demonstrated that expression of a mixture
of these olfactory gene messenger RNAs occurred only in cells
obtained from olfactory mucosa but not from brain, kidney,
liver, heart, lung, ovary, retina or spleen. Thus, a large family of
genes coded only for smell. Receptors are proteins having ligand
specificity and effector specificity.
Everything said explains why we perceive as odors the
various chemical substances present in the environment. The
first approximation to this question consisted in supposing
that on the surface of olfactory epithelial cells there had to be
receptors equipped with the ability to recognize odorants. The
binding of these molecules to receptors should stimulate them to
send signals to the brain. As Axel relates, these receptor proteins
would be the key to solving two basic dilemmas: does the system
rely on a few different receptors to respond to thousands of
different molecules or, conversely, are there a large number of
relatively specific receptors? And, furthermore, what is the way
the brain processes these responses to discriminate between
odors?
Using a complex set of molecular biology techniques, Axel
and Buck were able to identify in rats a large family of genes
with more than 1,000 different members- that is, they represent
between 2% and 3% of the total rat genes - which give rise to a
similar number of proteins that are those that act as olfactory
receptors. Although these receptor proteins have a fairly similar
structure - they belong to the family of G proteins that cross the
cell membrane - subtle differences between them are those that
confer the specificity to the odoriferous molecules. Unlike the
visual system that can distinguish thousands of colors using
three different types of receptors, the number of olfactory
receptors is comparatively huge. In humans, about 350 different
types of receptors have been identified, that is, the olfactory
world of a rat is infinitely richer than ours.
The other fundamental finding was the demonstration that
each receptor cell exhibits, on its surface, i.e., only one type
of receptor protein. Therefore, there must be at least as many
cells as possible receptors. It was verified that there are about
5,000 cells that exhibit on each surface of the receptor types. In
addition, it was demonstrated that each receptor has the capacity
to detect a limited number of odoriferous molecules, responding
to them with different intensity. In summary, each group of
olfactory epithelial cells is highly specialized in detecting a few
odorants.
The most astounding discovery of the team was finding that
there were so many odorant receptors. The 100 different genes,
which researchers first identified, were just the tip of the iceberg.
It now appears that there are between 500 and 1,000 different
receptor proteins, present in olfactory neurons of rat and mouse,
and probably in human neurons. Says Axel. “It’s 1 percent of the
genome”. “Most likely, the number of odorous substances far
exceeds the number of receptor proteins, in a ratio of at least 10
to 1,” says Axel. “In that case, how does the brain know what the
nose smells?”
Axel began by asking how many kinds of receptor proteins
are produced in a single olfactory neuron. “If an individual neuron expresses only a small number of receptors, or a single
receptor, then the problem of determining which receptors have
been activated, comes down to determining which neurons have
been activated,” he says. Concluded that a given olfactory neuron
can produce only one or a few odoriferous receptors. (Buck and
his colleagues have come to the same conclusion from their work
with mice).
The next step was to find out how these odorant receptorsand
the neurons that produce them-are distributed in the nose.
Also, with what parts of the brain do these neurons connect?
The different zones of the olfactory epithelium of the mice
are shown in red, blue and yellow. A different set of genes for
odoriferous receptors is expressed in each zone. When the
interaction between the molecule and the specific cell occurs, it
is activated by a complex cascade of chemical reactions. These
have been accurately characterized by both groups and in them
the formation of cyclic AMP and the subsequent opening of ion
channels. This generates a signal that is the one that travels,
through the thin processes of the neuroepithelial cells, to the
structures of the olfactory bulb known as olfactory glomerulus.
Receptors have a 7- transmembrane structure and
their activation produces, like all of their type, a cascade of
intracellular events that would start with G-protein activation
and increased cAMP levels (by activation of adenyl cyclase ) To
produce an opening of the ion channels and an activation of the
olfactory neurons.
The α subunit of the G proteins activates the adenylate
cyclase to catalyze the production of cAMP. CAMP acts as a
second messenger to open cationic channels. Inward diffusion of
Na+ and Ca2+ causes depolarization.
When studying DNA, we could determine that there are
1000 genes that code for 1000 different olfactory receptors,
each of which is expressed in millions of cells (almost every
cell in the organism carries a copy of each of the genes of each
being). However, the genes encoding olfactory receptor proteins
are active only in olfactory neurons. Taking into account that a
human contains approximately 100,000 different genes in their
DNA, it can be inferred that 1% of them are dedicated to smell.
According to a paper published in Nature Genetics, the
genes involved in the sense of smell are located in practice
on all chromosomes and represent 1% of the entire human
genome. However, the authors, from CNRS ERS of Montpellier
(France), add that 70% of these genes are not functional. They
are the results of a work whose objective is to determine how the
olfactory genes develop and if the sense of smell in the human
being has deteriorated over time as a consequence of evolution.
Using two types of genetic analysis, French scientists found
that the olfactory receptor genes are located on 19 of the 23
chromosomes. Through another method they found that olfactory
genes also exist in three other chromosomes. In an editorial of
the magazine it is said that the human being is able to recognize
more than 10,000 different odors, for which it is necessary the
existence of a large number of olfactory receptors and each of
them is related to a different gene. The study’s authors believe
that the human organism has 200 to 1,000 olfactory receptor
genes. They studied 87 amino acid sequences that appear
to be part of olfactory receptor genes. They found that 72%
were pseudo genes, or non-functional genes because they had
deteriorated as a result of mutations. “Same mutations,” they
wrote, “have been observed in man, chimpanzee and monkey,
suggesting that many of these genetic alterations occurred
during evolution in a common ancestor that may have existed
between 5 and 25 million years ago.” Another possibility is that
some olfactory receptor genes have been “silenced” by mutation
during evolution, but still exist in animals with a more developed
sense of smell. It’s what they try to find out with new research
on animals.
In 2016, Zozulya et al. published a study in the journal
Genome Biology describing that although there are more than
1,000 genes, only 347 olfaction receptor proteins are expressed.
In 2003, Gilad et al. Published in Proceedings of the National
Academy of Science (PNAS) that in humans more than 60% of
the olfaction genes are not functional (pseudo genes), unlike the
dog or the mouse in which the number of pseudo genes does
not exceed the 20%. The Axel and Buck groups found, in the
olfactory epithelium of the nose, that neurons that produce a
particular odoriferous receptor do not cluster; In contrast, these
neurons are randomly distributed within certain large regions of
the epithelium, called expression zones, which are symmetrical
on both sides of the animal’s nasal cavities. However, once the
axons reach the olfactory bulb, they are rearranged so that those
expressing the same receptor converge in the same place in the
olfactory bulb. The result is a highly organized spatial map of
information derived from different receptors. Surprisingly, the
spatial map is identical in the olfactory bulbs of all the mice that
were examined, says Buck. As she indicated, this information
provided the key to solving an ancient enigma.
The most interesting aspect of these studies consisted in
checking that recipient cells that possess the same type of
conscious of almost 10,000 different receptor smells on their
surface, send their processes to the same glomeruli. In this
way, maintaining the specificity, the information is transmitted
to other brain areas where it is combined with that from other
receptors, thus generating patterns that allow the recognition.
“The brain essentially says something like: I am seeing the activity
in positions 1, 15 and 54 of the olfactory bulb, corresponding to
the odorant receptors 1, 15 and 54, therefore, the perceived odor
must be that of the jasmine, Suggests Axel.
Other odors would be identified by different combinations of
this true alphabet of receptors. It is in this way that we build the
memory of what we have smelled. In other words, there is a kind
of labeled pathway extending from each receptor subtype to the
cerebral cortex which is thus kept permanently informed about the degree of activation of the various receptor types. Scientists
have long wondered how we can remember odors even though
each olfactory neuron, present in the epithelium, survives only
about 60 days, being replaced by a new cell. In most of the body,
neurons die without any successor. But as olfactory neurons die,
a layer of stem cells located beneath them; constantly generate
new olfactory neurons to maintain a constant supply.
“The mystery was, how do we remember smells when these
neurons are constantly being recycled and the new batch has to
form new synapses?” Says Buck. “Now we know the answer: the
memories survive because the axons of the neurons expressing
the same receptor always go to the same place. Binding
proteins also act as true landfills by inactivating the molecules
in question, once the signal transduction has been performed,
thus releasing the receptor who will be able to receive a new
molecule. This DETOXIFICATION of molecules is achieved by the
ligand- receptor protein phosphorylation, which is performed
by two pathways. The P- 450 cytochrome mono-oxygenase
pathway, which acts by hydroxylation of the substances involved
and the pathway of the UDP glucoronyl transferase that catalyzes
the conjugation of glucuronic acid. Olfactory perception is
cataloged as an aesthetic sense, capable of producing emotions
and memories, which in turn produce different thoughts and
behaviors. The human vomeronasal system is the detector of
“vomeroferins”, substances that induce changes in maternal and
sexual social behavior.
The term “vomeroferin” is preferred to refer to those
substances which, by acting primarily on receptors of the
vomeronasal organ of terrestrial vertebrates, especially
mammals, induce changes in maternal and sexual social
behavior. The nasal vomer organ (OVN) maintains connections
with the hypothalamus and the limbic system, but is not known
cortical representation. In the human species, stimulation of
VNV with vomeroferins produces behavioral changes, along with
impressive neuroendocrine changes.
Binding proteins also act as true landfills by inactivating
the molecules in question, once the signal transduction has
been performed, thus releasing the receptor that will be able
to receive a new molecule. This DETOXIFICATION of molecules
is achieved by the ligand- receptor protein phosphorylation,
which is performed by two pathways. The P- 450 cytochrome
mono-oxygenase pathway, which acts by hydroxylation of the
substances involved and the pathway of the UDP glucoronyl
transferase that catalyzes the conjugation of glucuronic acid.
Olfactory perception is cataloged as an aesthetic sense, capable
of producing emotions and memories, which in turn produce
different thoughts and behaviors. The human vomeronasal
system is the detector of “vomeroferins”, substances that induce
changes in maternal and sexual social behavior. The term
“vomeroferin” is preferred to refer to those substances which,
by acting primarily on receptors of the vomeronasal organ of
terrestrial vertebrates, especially mammals, induce changes
in maternal and sexual social behavior. The nasal vomer organ
(OVN) maintains connections with the hypothalamus and the
limbic system, but is not known cortical representation. In the
human species, stimulation of VNV with vomeroferins produces
behavioral changes, along with impressive neuroendocrine
changes.
Given the frequency with which the otolaryngologist and the
plastic surgeon surgically intervene the nose, it is essential to
know the Nasal Vomer System to avoid its injury, and to know
the anatomical-functional data of its activity, which confer the
human Vomere-nasal system the Characteristic of a sensory
system. As a corollary, and for now let’s say that love comes
through the smell. It is possible that the male-female attraction
may be pheromonal. Pheromones are found in the secretion
of the axillary glands and the dermal surface. It is possible to
conclude that the sexual attraction enters the eyes, but for
that “chemistry between the sexes” to occur, the responsibility
corresponds to the pheromones.
To know more about Open Access International
Journal of Pulmonary & Respiratory Sciences please click on: https://juniperpublishers.com/ijoprs/index.php
To know more about Open access Journals
Publishers please click on : Juniper Publishers
To know more about juniper publishers: https://juniperpublishers.business.site/
Comments
Post a Comment