Recent Approaches for Novel Treatment for Pulmonary Diseases-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS INTERNATIONAL JOURNAL OF PULMONARY & RESPIRATORY SCIENCES
Abstact
Pulmonary drug delivery system (PDDS) remains an
important route for administration of different types of drugs.
Pulmonary route has been concerned for scientific and biomedical
importance in past years for treatment of lung diseases. Drug delivery
by the pulmonary route has evolved to be one of the most widely used
systemic or local drug delivery approach. The drug delivery systems for
the treatment of lungs diseases are increased due to their prospects for
localized therapy in the lungs. Pulmonary route as one important aspect
which makes a possibility to deposit drugs more site-specific and
targeting of drugs, as well as enhancing the local drug activity while
reducing systemic side effects and first- pass metabolism. This review
detailed discusses the physiological (technical) and efficacy aspects of
the novel pulmonary route of drug targeting system. The review also
focused on the mechanisms of pulmonary drug administration along with
compatibility of the excipient employed, uses of the various devices,
and new techniques of particulate dosage manufacturing. Hence, the
better understanding of complexes and object facing the development of
PDDs offer an opportunity to the pharmaceutical scientist in minimizing
the clinical and technical gaps.
Introduction
Pulmonary drug delivery system has been widely used
for the treatment of lung diseases and is acclaimed for the asthma
treatment and chronic obstructive pulmonary diseases. This system is a
needle free technique. The origin of inhaled therapy seen in 4000 years
ago in India, where patient smoked the Atropa belladonna leaves to
suppress cough. In the early 20th century, asthmatics smoked asthma
cigarettes that contain stramonium powder mixed with tobacco to treat
the symptoms of their disease [1].
But administration of drug by this route is technically challenging
because oral deposition can be high, and variation inhalation techniques
may affect the quantity of the drug delivered to the lungs. Delivery of
locally acting drugs to the site of action reduce the amount of dose
needed to produce the pharmacological action but now the lungs have been
studied as a possible route to administer the treatment of systemic
disease like diabetes mellitus, angina pectoris, cancer, bone disorders,
migraine, tuberculosis, acute lung injury and others. Pulmonary
delivery is apprehended by various ways like aerosols, Metered dose
inhaler systems (MDIs), Dry powder inhalers (DPI) and Nebulizers. These
types of system may contain Nano formulations like microemulsions,
micelles, bio-degradable nanoparticles and liposomes.
According to the need we have numerous types of
dosage forms like controlled release, sustained release or immediate
release. The sustained release dosage forms are favoured over the
uncoated, immediate release due to the same reasons like increase in the
local effects on the use at the site of infection, need of relatively
small doses for the effective therapy and reduction in systemic exposure
due to local application leads to reduction of adverse effects. Some
scientists are annoying to evaluate the use of a sustained release form
of inhaled rifampin for TB therapy. The pulmonary drug delivery field is
efficacious, stupendous and advanced technique in today's applied
pharmaceutical research [2,3].
It includes a wide variety of persistent pulmonary
disorders, such as asthma, chronic obstructive pulmonary disease (COPD),
cystic fibrosis, pulmonary tuberculosis, idiopathic pulmonary fibrosis
(IPF) and lung cancers [4,5].
Some of these diseases are irreversible and often fatal, and no
treatments have been shown to be effective for completely restoring lung
functions. Approximately 300 million and 210 billion people in the
world are currently estimated to suffer from the two most prevalent
diseases, asthma and COPD, respectively [6,7]. Pulmonary tuberculosis is also a frequently found infectious disease with 8.6 million chronic case reported in 2012 [8,9].
Traditional pharmacotherapy for chronic lung diseases can be classified
into different types according to forms of therapeutic agents. A
variety of chemical drugs, peptides, antibodies, and genetic molecules
(eg. SiRNA, shRNA and miRNA) have been employed to treat the chronic
lung diseases [10-12].
Unfortunately, most of chronic lung disease cannot be completely cured
by pharmacotherapy alone. In cases of asthma, controlling the symptoms
is the only available current option. Likewise, steroids,
bronchodilators, pirfenidone, and nintedanib are currently used for the
management of COPD or IPF, but no effective treatments are available to
fully cure these types of diseases [13].
It is a blockage of an artery in the lungs by a
substance that have travelled from elsewhere in the body through the
bloodstream. Pulmonary embolism usually results from a blood clotting in
the leg that moves to the lung. The risk of blood clotting increased by
cancer, prolonged bed rest, smoking, stroke, obesity, pregnancy and
after some types of surgery. Some cases are due to the embolization of
air, fat or amniotic fluid. Diagnosis is based on signs and symptoms in
combination with test results. Diagnosis is based on signs and symptoms
in combination with test results. If the risk is low, a blood test
'D-dimer' may rule out the condition. Otherwise a perfusion scan or
ultrasound of legs may confirm the diagnosis. Together deep vein
thrombosis and PE are known as venous thromboembolism. Because pulmonary
embolism almost always occurs in conjunction with deep vein thrombosis,
most doctors refers to the two conditions together as venous
thromboembolism. Signs include low blood oxygen levels, rapid breathing,
rapid heart rate and sometimes a mild fever. Severe cases can lead to
passing out, abnormally low blood pressure and sudden death. Symptoms
may also include breath shortness, chest pain upon breathing in, and
coughing up blood. Symptoms of a blood clot in the leg may also be
present such a red, warm, swollen and painful leg.
a. Helps in minimizing the dose of API molecule used
for oral routes i.e. drug content of one 4mg tablet of salbutamol equals
to 40 doses of meter doses [14].
b. Degradation of drug by liver is avoided [15].
c. Onset of action is very rapid and provides local action within the respiratory tract [16].
d. Reduces extracellular enzyme levels compared to GI tract due to the big surface area of alveolar [17].
e. It is needle free pulmonary delivery [18].
f. Inhaled drug delivery puts drug where it is needed [19].
g. Allows for a reduction in systemic side-effects.
h. Reduces evasion of first pass hepatic metabolism by absorbed drug.
i. Inhaling helps to avoid gastrointestinal tract
problems such as poor solubility, low bioavailability, gut irritability
unwanted metabolites, food effects and dosing variability.
j. It provides a non-invasive method of delivering
drugs into the bloodstream for those molecules that can only be
delivered by injection. These include proteins and peptides, such as
insulin for diabetes or interferon beta for multiple sclerosis and many
of the drugs developed in recent years by biotechnology companies [14,18].
a. Drug absorption may be limited by the physical barrier of the mucus layer.
b. Patient may have some problems using the pulmonary drug delivery devices correctly.
c. Oropharyngeal deposition gives local side effect.
In the course of the most recent decade, the systemic
absorption ofa board scope oftherapeutic agents after pulmonary
application has been shown in animals and also in people. Through
pulmonary course, the medication can be controlled by two essential
modes; to start with, intranasal organization, which has anatomical
impediment, for example, narrows airway lumen, second, oral inhalative
organization. By oral inhalative organization obviously better outcomes
can be normal as it permits controlling small particles with a
concentration loss of just 20% in correlation with 85% by nasal route.
Oral inhalative organization can again be delegated intratracheal
instillation and intratracheal inhalation. The most well-known technique
utilized as a part of research facility is the intratracheal
inhalation. In the intratracheal instillation, a little measure of
medication arrangement or scattering is conveyed into the lungs. The
most well-known strategy utilized as a part of labs is the intratracheal
instillation. In the intratracheal instillation, a little measure of
drug solution or dispersion is conveyed into the lungs by an exceptional
syringe. This gives a quick and quantifiable technique for drug
conveyance to the lungs. The localized drug deposition is accomplished
with a relatively small absorptive range. In this way, the instillation
procedure is much basic, non-costly, and has non uniform drug
distribution. In preclinical creature (animals) studies intratracheal
instillation has as often as possible been utilized to evaluate the
pulmonary absorption and systemic bioavailability, particularly as to
the exact dosing and viability related with this technique. However,
intratracheal instillation is not a physiological route for application,
and results got from these reviews may not be transferable to aerosol
applications in people.
Unexpectedly, inhalation strategy utilizes aerosol
system by which we can get more uniform distribution with incredible
penetration. However, this technique is all the more expensive and hard
to quantify the correct dose in lungs. The deposition of drug by aerosol
organization in the pulmonary airway route mainly has three systems: -
gravitation, sedimentation, inertial impactation, and diffusion. If the
drug molecule size is nearly greater, then, deposition happens by
initial two mechanisms where, either sedimentation happens because of
gravitational force or inertial impaction happens because of
hyperventilation. At the point when the molecule size is littler they
deposit mainly by diffusion mechanism, which in turn is depends on the
Brownian movement. Aside from the pulmonary morphological aspects and
ventilatory parameters size of the particles or droplets and the
geometry is very essential. The size of particles or droplet in term of
diameter along with the surface electrical charges, shape of the
particulate matter it is a fiber and hygroscopy likewise having
significant impact on drug deposition through pulmonary route. The term
mass middle aerodynamic diameter is utilized and it relies on upon size,
shape, and thickness of the particulate system.
Low Efficiency of Inhalation System
The major challenge in pulmonary drug delivery is the
low efficiency of presently available inhalation systems. Ideal aerosol
particle size is very important for deep lung delivery (pulmonary drug
delivery). Since if the particles are too small, the optimum particle
size for deep lung deposition is 1-5 mm, they will be easily exhaled,
and if the particle size is too large, they effects on the oropharynx
and larynx.
Less Drug Mass Per Puff
To get adequate effects by the pulmonary drug
delivery system the delivery of many drugs which require milligram doses
but with most existing systems, the total amount of drug per puff
transferred to the lower respiratory tract is too low (less than
1000mcg).
Poor Formulations Stability for Drugs
Most traditional small molecule asthma drugs are
crystalline in nature, comparatively moisture resistant in the dry
molecules. Whereas in the case of corticosteroids, which are unstable in
liquid state, amorphous, and highly moisture sensitive in the dry
state.
Improper Dosing Reproducibility
The main reason for poor dosing reproducibility is
worsening of diseases, problem in device, and instability of
formulation. To get maximum dose reproducibility patient education play
important role.
Various factors that may affect pulmonary absorption rate and bioavailability are listed in Table 1.
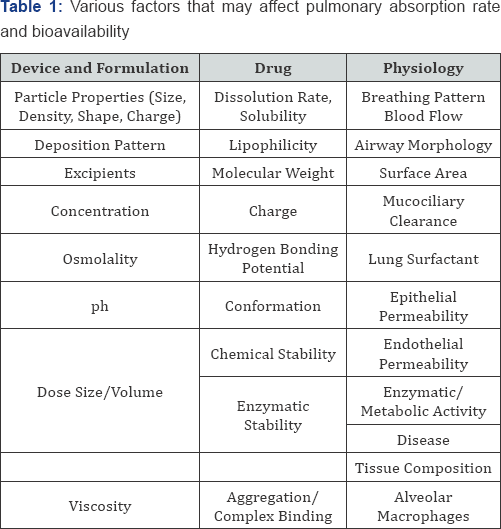
Factors which Affecting Absorption and Bioavailability ff Pulmonary Drug Delivery System [21]
Formulations
The drugs may be inhaled by pulmonary route utilizing
two techniques: aerosol inhalation and intratracheal instillation. By
applying aerosol technique, we could obtain more uniform distribution
with maximum extent of penetration into the peripheral or the alveolar
region of the lung, but this costs more and also faced with difficulty
in measuring the exact dose inside the lungs. In contrary to this,
instillation process is much simple, not expensive and has non-uniform
distribution of drugs [22].
Following types of inhalation devices are present:
Nebulizers
There are two types of nebulizer systems which are
widely used as aerosolize drug solutions or suspensions for drug
delivery to the respiratory tract i.e. the ultrasonic nebulizer and the
air jet nebulizer. In ultrasonic nebulizers, ultrasound waves are found
in an ultrasonic nebulizer by a ceramic piezoelectric crystal that
vibrates when electrically excited. The aerosol produced by an air jet
nebulizer is generated when compressed air is forced through an orifice;
an area of low pressure is formed where the air jet exists. Nebulizers
are useful for the treatment of acute asthma and emergency care unit or
for treating patients with severe asthma at home. The nebulizer can
transport more drugs to the lungs than metered dose inhalers (MDI) or
dry powdered inhalers (DPI), the most common disadvantage of nebulizer
are lack of possibility, higher costs of drug delivery as a result of
the larger need for assistance from health care professionals, and the
need for higher drug doses to achieve a therapeutic result [2,4,23,24].
a. Recent advancements in liquid aerosol innovation
consolidate the advantages of MDIs and nebulizers are called metered
dose liquid inhalers. The big advantage that every one of these systems
focus for the reduced velocity of the aerosol. Liquid inhalers applying
the idea of a low velocity aerosol are frequently alluded to as 'soft
mist inhalers'.
b. Wet nebulisation goes for the era of mono
disperses aerosols. The nonattendance of propellants in the formulation
by applying aqueous drug formulated causes a reduction in the residual
volume after nebulisation and an enhanced portability compared and
nebulizers.
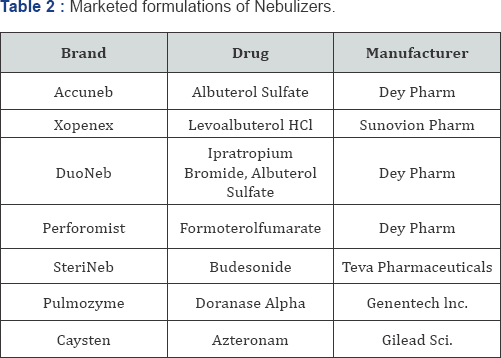
c. Now-a-days various marketed formulations of nebulizers are available in market. Some of such formulations are listed in Table 2.
Metered Dose Inhalers (MDIs)
MDI is a complex system designed to provide a fine
mist of medicament, generally with an aerodynamic particle size having
less than 5 microns, for inhalation directly to the airways for the
treatment of respiratory diseases such as asthma and COPD [1,25].
They can be given in the form of suspension or solution. They consist
of a micronized form of the active pharmaceutical ingredient (API) in a
propellant under pressure with surfactants to prevent clumping of API
molecule. Lubricants for the valve mechanism and other solvents are the
other constituents. When the device is pressed, the propellant gets
disclosed to atmospheric pressure, which leads to aeroionisation of the
drug. As it travels through the air, the aerosol warms up leading to
evaporation of the propellant that reduces the particle size to the
specific range. The fraction of drug to the airways ranges from 5-15% [26].
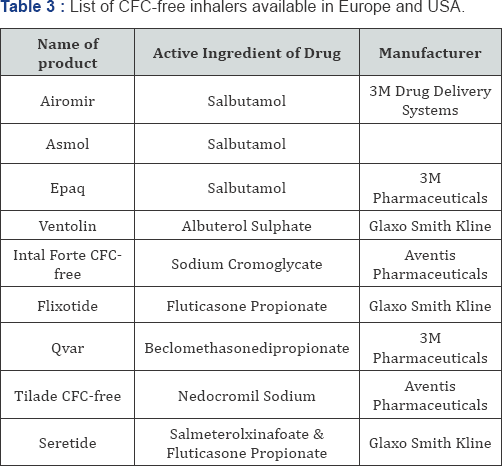
In 1990, attempts were actively made of reformulate MDIs as a result of
mandatory ban on the use of propellant chlorofluorocarbons, which have
been concerned in the depletion of the Earth's ozone layer. Optional
propellants, such as hydrofluoroalkane 134a (HFA-134), have be
considered for their potentials to change CFCs since 1990. CFC-free
inhalers formulations those are available in Europe and USA are listed
in Table 3.
a. There has been much importance in the differences
in effects of enantiomer of many medications and beta agonist adrenergic
bronchodilators have received much attention. Recently levo salbutamol
is present in the market.
b. Use of spacers to improve patient coordination
with metered dose inhalers. Evidence indicates considerable intra and
inter-subject variability for the inhalation.
c. The auto-haler TM is the first breath activated
pressurized metered dose inhaler. Auto-haler solve the key problems of
the pressurized metered dose inhaler (pMDI), does not rely on the
patient's inspiratory efforts to aerosolize the dose of medication
unlike dry powder inhalers.
Dry Powdered Inhalers (DPIs)
Dry powder system contain micronized drugs single or
its mixes with a reasonable carrier, essentially as lactose, for
conveyance to the lungs, which anticipates aggregation and increase flow
properties of drugs [4,25,26].
When the patient activates the DPI and inhalers, airflow through the
device makes shear and turbulence; air is brought into the powder bed
and static powder mix is fluidized and enters the patient’s airways.
There, the drug particles isolate from the carrier particles and are
carried deep into the lungs, while the larger carrier particles affect
in the oropharynx and are cleared. Along these lines, deposition into
the lungs is controlled by the patient's variable inspiratory airflow.
The three principle variables Drug, Carrier, and device may influencing
the act of pulmonary delivery of drug. In addition, DPIs are small,
versatile device that can be effortlessly conveyed in a satchel or
pocket. These devices are generally acknowledged inhaled delivery dosage
form, especially in Europe, where they are at present utilized by
roughly 40% of asthma patients. Absence of the necessity of propellant
is leverage of DPIs over MDIs [27].
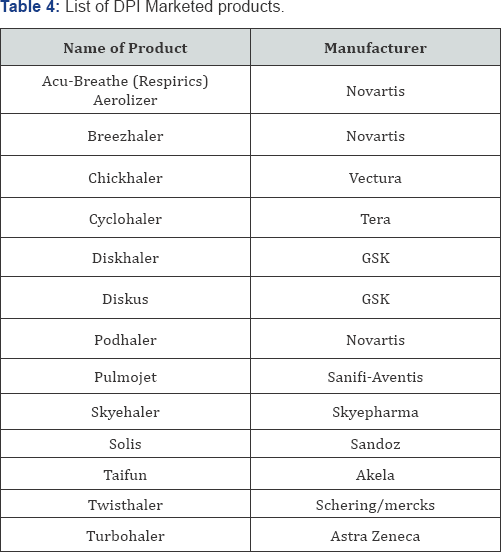
The fraction of the drug delivered to the site of activity by a DPI
differs from 9% to 30%. As of now there are two sorts of MDIs [4,25,28]. Different advertised items accessible in the market are shown in Table 4.
a. Larger drug available for pay loads per puff.
b. Blending is not requiring for it.
c. Enables use of small, flow-rate independent inhalers.
d. The particles readily disaggregate despite their small size.
e. Enables improved lung deposition, dose decrease variability and potential for condensed dose through enhanced dispersibility.
a. DPIs Performance can be adjusted through changes
in the design of the device and furthermore changes in the powder
formulation and designing. The strengths required in the process prompts
the particle-particle interactions in the agglomerates and furthermore
the forces playing a part in the de-agglomeration process.
b. Supercritical fluid technology (SCF) is connected to enhance the surface properties of the drug substance.
c. Large porous particles have decreased
inter-particulate forces because of their low density, the irregular
surface structure or reduced surface free energy. Furthermore, these
particles are accounted for to have enhanced aerodynamic behaviour in
the airways, while phagocytises of the stored particles in the alveoli
is lessened. In another approach, smaller porous particles (3-5 mm) have
been utilized to enhance de-agglomeration and lung deposition.
d. Air classifier Technology has been recently utilized as a part of the devices to avoid agglomeration in devices.
Current Trends
Inspiromatic is delivered by Inspiro Medical, a
portfolio organization of the Trendlines bunch, and is intended to
replace hard-to-utilize nebulizers for youthful kids and additionally
the elderly and individuals with specific handicaps. Inspiromatic has an
internal microcontroller and flow sensor that identifies the ideal time
to deliver the medication and consequently disperse the drug particles
in the correct size without requirement for forceful Inhalation [32].
Twisthalers
This inhalation device is generally free of flow
rates. It has been exhibited that with inspiratory flow between 28 l/min
and 60 l/min 91% to 112% of the metered dose is delivered at the
mouthpiece. The fraction of particles smaller than 6.5 μm amount up to
40% at an inspiratory flow of 60 l/min. moreover drug doses released
from the Twisthaler® just change somewhat between each dosage. The twist
haler is endorsed by momentosonefuroate [33].
Nexthaler
Cambridge Consultants is attempting to develop the
upcoming era of dry powder inhaler for ChiesiFarmaceuticiSpA, a rising
European pharmaceutical organization situated in Parma, Italy. The new
inhaler intends to be the most straightforward to- utilize dry powder
inhaler available. The outline incorporates features to enhance
performance and make the device discreet, natural to utilize and
futuristic in appearance [34].
HandiHaler
It is utilized to deliver the substance of Spiriva
inhalation Capsules containing the bronchodilator tiotropium, utilized
for long-term treatment of chronic obstructive pulmonary disease (COPD)
and other obstructive airways infection; to mitigate side effects of
bronchospasm [35].
Turbuhalers
It has the dry powder pharmaceutical inside the
tube-molded inhaler. They have a removable cover and a contorting base.
It is a 'breath-activated' device means the dry powder medication is
"sucked" from the device instead of "fired" like it is from different
devices. Turbuhalers might be hard to use for young kids, or grown-ups
who are short of breath. It is prescribed to have a puffer and spacer
accessible for crises [36].
Easy Haler
The Easy haler®, is produced and patented by Orion,
is an environment friendly and efficient, simple to use for the
treatment of respiratory sicknesses, for example, asthma and chronic
obstructive pulmonary disease (COPD). Orion aims to expand the product
family of inhalable Easy haler® drugs utilized for treating asthma and
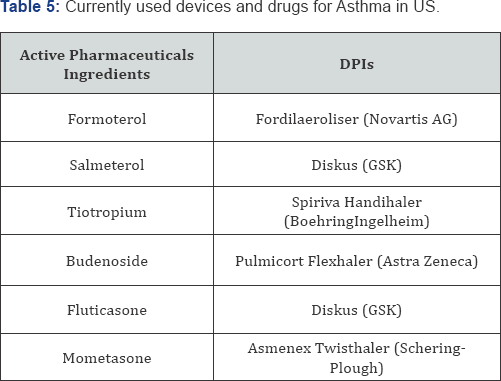
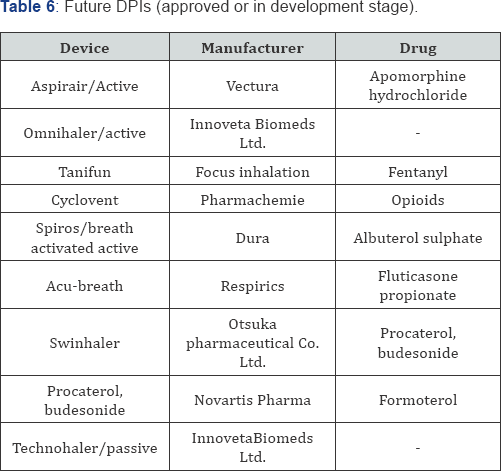
COPD [37]. Currently used delivery devices and drugs utilized inhaled drugs for asthma in US is shown in Table 5 and future improved formulations of DPIs are shown in Table 6.
Several models are presented for preclinical
investigation of pulmonary drug absorption and deposition. The
complexity of the models range from permeability screening experiments
in cell culture models to in vivo pharmacokinetic analysis in animals.
The design of the experiments comprises both selection of the most
relevant biological model for the specific issue, and the selection of a
drug delivery system i.e. appropriate for the amount of test material
available and that can selectively deposit a defined dose of the drug to
the intended lung region. A combination of in vitro and in vivo models
is needed to elucidate the mechanisms, rate, and extent of absorption,
as well as the distribution, metabolism and elimination of a drug after
pulmonary administration [1].
Cell Culture Models
The inaccessibility and heterogeneous composition of
the airway epithelium makes it difficult to mechanistically evaluate
pulmonary cellular integrity and physiological function. For
investigation of drug transport mechanisms, precise dosing and sampling,
as well as defined local drug concentration and surface area of
exposure, are important parameters that need to be controllable and
reproducible. Therefore, a variety of airway and alveolar epithelial
cell culture models of animal and human origin have been established as
in vitro absorption models. The models include both continuous cell
cultures, primary cell cultures and air-interface cultures [38].
Continuous Cell Cultures: They are
reproducible and easily utilize than primary cell cultures but they
frequently don't have the differentiated morphology and the biochemical
characteristics of the original tissue. There are a small number of cell
lines resulting from alveolar epithelial cells. A549 is a type II
alveolar epithelial cell line that originates from human lung
adenocarcinoma. It can be very helpful in metabolic and toxicological
studies but it is less interesting as a drug delivery model because A549
cells do not form stretched monolayer's [39] .
Primary Cell Cultures: The primary cell
cultures more closely resemble the native epithelia and used as model
for pulmonary drug delivery, but are less reproducible and more time
consuming to work with compared to the cell lines, which make them less
suitable for permeability screening purpose. Type II pneumocytes for
primary culture can be removed from the lung. Human cells are the mainly
representative of the clinical circumstances, but they are less
available than cells from other mammals. Human type II pneumocytes are
removed from normal lung tissue of patients undergoing partial lung
resection. In culture, the cells experience segregation into type I-like
cells, as indicated by morphological and histochemical change. In
premature stages of the cell culture, the cells create elevated levels
of surfactant protein C and little levels of caveolin 1, a marker of
type I pneumocytes, and on the other hand at later stages. On day 8 of
culture, the cells form a tight monolayer consisting mainly of type I
cells and some interspersed type II cells, with TEER > 2000 Ω cm 2
and potential difference > 10 mV [40] .
Air-Interface Cultures: AIC models are
permitting aerosol particles to place straight onto semi-dry apical cell
surface. Drug deposition and dissolution take place in a small volume
of cell lining fluid, a circumstances that mimics more directly
deposition on the lung surface in vivo. The AIC show greater similarity
to airways epithelial morphology, with glycoprotein discharge, more
prominent microvilli and the construction of a pseudo stratified layer
of columnar cells, while the liquid- covered culture created a monolayer
of cells.
In Vivo Animal Models
In vivo pharmacokinetic experiments in animals
provide data on the fate of a drug and its metabolites in the body by
assessment of the drug concentration in plasma or tissues. Drug exiting
device Inhaled dose Systemic exposure Alveolar deposition Pulmonary
deposition Tracheal/Bronchial deposition First- pass metabolism/ Not
absorbed from GI-tract Extra pulmonary deposition Exhaled dose
Distribution/Metabolism/Elimination Absorbed AM (phagocytosis by
alveolar macrophages) MC (Mucociliary clearance metabolism). By
contrast, small rodents, mice models for preliminary studies on
pulmonary drug delivery because they can be used in large numbers. Mice
have been used less often for assessing pulmonary release of
systemically performing drugs because pharmacokinetic studies are not
optimally perform in mice.
Owing to its small size, one mouse can offer only one
blood sample at a time (1 ml whole blood sample is withdrawn by cardiac
puncture and mouse euthanasia must be done at each time point of the
plasma drug concentration-time curve. Guinea pigs have been generally
used as an animal form of allergic asthma and infectious diseases (e.g.,
tuberculosis) since the airway anatomy and the respond to inflammatory
stimuli are similar to the human case. The dissimilar mammals do not
show to present related Mucociliary clearance and alveolar macrophage
morph metric. In large mammals, the rate of mucus permission in
millimetres per minute is elevated compared with small rodents. Though,
huge mammals also have longer airways than minute rodents and thus,
worldwide, the bronchial permission of inhaled particles is
comparatively slow in humans (> 24 h).
Cascade Impactors
It determines the aerodymic activity of aerosol
particles by size-separating the dose in impactor plates. It gives up
valuable aerosol parameters such as the fine particle fraction, mass
median aerodynamic diameter. In vitro particle sizing data obtained from
impactors plan first at scheming the quality of the pharmaceutical
product and next at provide an analysis tool for product improvement. It
is projected that outcome from cascade impactors forecast human lung
deposition data as particle aerodynamic size determine the deposition of
aerosol in the respiratory tract.
Passive Inhalation
During the inhalation of aerosolised drugs, all the
mammals are kept awake and allowed to breathe normally. Aerosolised
drugs are delivered using an aerosolisation chamber in whole body
exposure systems, head-only or nose-only exposure systems. The devices
is generally used for generating aerosols are nebulizers. Passive
inhalation is principally used in the mouse and less frequently in heavy
animals (rat, guinea-pig, dog). This method is more representative of
drug delivery to the human lungs than intratracheal instillation of high
volumes of liquids. The drug concentration in the aerosol is determined
by sampling the test atmosphere and quantifying the drug in the sample.
Whole Body Exposure Systems
In this system, animals are placed in a sealed
plastic box that is connected to a nebuliser or a generator of dry
powder aerosol. Although this system allows a less stressful pulmonary
drug administration to an important number of animals, there is
potential drug absorption across the skin after deposition on the animal
fur, from the nasal mucosa and from the gastrointestinal tract.
Head-Only or Nose-Only Exposure Systems
In these systems, the animal is attached to the
exposure chamber and only the nose or the nose is in contact with the
aerosol device. The systems can be designed for delivering drugs to one
or to several animals. Compared with the whole body exposure system, the
head-only or nose-only exposure systems offer different advantages. The
low volume of the aeroionisation chamber reduces the amount of drug
needed to generate the aerosol.
Intranasal administration
This is mostly known for local drug delivery to the
nasal mucosa but it can also be used for intrapulmonary drug
administration in mice. It is performed on the anaesthetised mouse kept
in a vertical position. With the help of a micropipette, the solution is
deposited on a nostril and is simply aspirated in respiratory airways
during breathing. Use of a small volume of solution restricted drug
administration to the nasal cavity but that the use of a larger volume
of solution allowed a deeper administration to be reached in lung upper
airways.
Recent Advancements In Formulation of Pulmonary Drug Delivery
Effective inhalable meds are formed by drug
formulation and designing. Formulation stability is another trouble in
producing pulmonary drug delivery. Formulation is in charge of keeping
drug in pharmacologically active state, it must be proficiently
delivered into the lungs, to the fitting activity on the specific site
and stay in the lungs until the coveted pharmacological effect happens.
Here a formulation that is retained in the lungs for the proper time
allotment and avoids the clearance mechanisms of the lung may be
necessary. A few factors have been incorporated into support of
developing nasal formulations containing liposomes, microspheres and
nanoformulations for intranasal drug delivery. In fact, it is not clear
if those formulations increase drug absorption by transporting
encapsulated drug across the membrane or just because they improve the
nasal retention time and stability of the drug. However, their
utilization is in extensive growth and the outcomes have been
exceptionally skilled.
Prodrugs
Prodrug is used to explain compounds that undergo
biotransformation prior to exhibiting their pharmacological effect.
Throughout the years, prodrugs have been used to decrease the bad taste
of the drug, poor solubility, insufficient stability, incomplete
absorption across biological barriers and metabolism to inactive or
toxic species. Intranasal drugs are can administered as solutions or as
powder form which need to undergo a dissolution process before
absorption. Lipophilic drugs easily diffuse through the bio membranes,
but they are poorly soluble in water. So they have to be administered as
a prodrug with higher hydrophilic character in order to make possible
the manufacturing of an aqueous nasal formulation with a desirable
concentration. Once in the blood stream, the prodrug must be quickly
converted to the parent drug. In contrast, very hydrophilic polar drugs
may not have ability to cross biomembranes. Thereby, if they are
administered as prodrugs with higher lipophilic character, the
penetration through the membrane may increase. For instance, L-Dopa is
poorly soluble in water, so it is very difficult to develop a
corresponding intranasal aqueous formulation with an effective dose.
Produced various prodrugs of L-Dopa and observed that their solubility
enhanced significantly in contrast with the pure drug, allowing, hence,
the development of adequate nasal formulations. Furthermore, their nasal
administration resulted in an accelerated and complete absorption to
the systemic circulation, where quick conversion to L-Dopa takes place.
Similar report was obtained for testosterone which is also poorly
soluble in water [41,42].
Enzymatic inhibitors
Nasal mucosa and nasal mucus layer have a huge
variety of enzymes. Hence they act as enzymatic barriers for nasal drug
delivery. Various approaches have been used to avoid enzymatic
degradation, including the use of proteases and peptidases inhibitors.
For example, bestatine and comostate amylase are used as aminoptidases
inhibitors and leupeptine and aprotinin as trypsine inhibitors probably
involved in the degradation of calcitonin. Furthermore, bacitracin,
amastatin, boroleucin and puromycin have been used to avoid enzymatic
degradation of drugs such as leucineenkephalin and human growth hormone [41].
Absorption enhancers
Hydrophilic drugs may be poorly permeable across the
nasal epithelium and may show an insufficient bioavailability and
therapeutic efficacy. Their absorption is greatly improved by
administered in combination with absorption enhancers which induce
epithelial barrier by modifying the phospholipidic bilayer. In
intranasal drug delivery, absorption enhancers most used are surfactants
(laureth-9), bile salts, fatty acids (taurodihydrofusidate) and
polymeric enhancers (chitosan, cyclodextrins, poly-Larginineand
aminatedgelatin)[42-46].
Novel drug formulations
Several factors have been included in support of
developing nasal formulations containing liposome, nanoparticles and
microspheres for intranasal drug delivery. In fact, it is not clear if
those formulations increase drug absorption by transporting encapsulated
drug across the membrane or just because they increase the nasal
retention time and stability properties of the drug.
Liposomes: Liposomes are a type of vesicles
that contains both of numerous, few or only one phospholipid bilayers
enclosing one in or more aqueous compartments in which drugs and
different substances may be put stored. Presently a day, they have been
examined as a vehicle for sustained release therapy in the lung disease
treatment, gene therapy and as a technique for delivering therapeutic
agents to the alveolar surface for the treatment of systemic diseases.
Liposomal drug delivery systems introduce different focal points, for
example, the effective encapsulation of small and large molecules with
an extensive variety of hydrophilicity and pKa values. In fact, they
have been found to enhance nasal absorption of peptides, for example,
insulin and calcitonin by expanding their membrane penetration. This has
been ascribed to the expanding nasal retention of peptides, insurance
of the entrapped peptides from enzymatic degradation and mucosal
membrane disruption. Incorporated insulin in liposomes coated with
chitosan and carbapol and administered them intranasally to rats. The
report demonstrates that this formulation was effective and that its
mucoadhesive property is a reasonable choice for a sustained release of
insulin [24,47,48].
Nanoparticles: Nanoparticle systems are being
investigated to improve drug delivery and intranasal drug
administration. Nanoparticles are solid particles having a diameters
ranging from 1-1000 nm. They consist of macromolecular materials and
which are therapeutically used as adjuvant in vaccines or as drug
carriers, in which the active substance is entrapped, dissolved,
encapsulated, adsorbed or chemically attached. Nanoparticles have
several advantages due to their Nano size property, but only the small
nanoparticles is able to penetrate the mucosal membrane by Para cellular
route and in a limited quantity, since the tight junctions are in the
order of 3.9-8.4 Å. There are several studies have been suggested that
nanoparticle systems can be preferably suited as a vehicle for sustained
release therapy. Sustained release from therapeutic aerosol can prolong
the residence of an administered drug in the airways or alveolar
region, minimize the risk of adverse effects by decreasing its systemic
absorption rate, and increase patient compliance by reducing dosing
frequency. Nanoparticle systems are also suitable for the delivery of
nasal vaccines [41,49].
Nanoparticles are characterized as particles with
submicron sizes in diameter. Since the mid-2000s, a wide assortment of
nanoparticles has been produced as drug carriers for biomedical
applications. Different materials have been utilized for the manufacture
of nanoparticles. Nanoparticles regularly share their unique physical
properties due to their submicron estimate, and these characteristics
have been exploited for disease-specific drug delivery. Significantly,
nanoparticles have a bigger surface area than micro materials with
similar total masses. It permits nanoparticles to have a superior
opportunity to have contact with the surrounding tissues and cells,
subsequently increasing the efficiency of cellular delivery. Moreover,
systemically injected nanoparticles in vivo generally show enhanced
accumulation to the pathological lesions found in tumours, hemorrhagic
diseases, and inflammatory diseases. These findings suggest that
nanoparticles have perfect properties to be utilized as imaginative
carriers for drug delivery.
Microspheres
This technology has been useful in designing of nasal
drug delivery formulations. Microspheres are generally based on
muco-adhesive polymers like chitosan, alginate, which provide various
advantages for intranasal drug delivery. Moreover, microspheres also
protect the drug from enzymatic metabolism and gives sustain drug
release, thereby prolonging its effect [41].
Mucoadhesive Drug Delivery Systems / Mucoadhesion
MCC is one of the very important limiting steps for
nasal drug delivery, because it decreases the time allowed for drug
absorption. So these systems improving the nasal drug absorption, and
also prolonging the contact time between nasal mucosa and drug.
Mucoadhesion represents the attachment of the drug delivery to the
mucus, involving an interaction between mucin and a synthetic or natural
polymer known as mucoadhesive. The sequential events that occur during
this mucoadhesion include different steps. First mucoadhesive systems
absorb water from mucus layer and get wet and swell. Following this, the
polymer intimately penetrates into the mucus and, hence, localizes the
formulation in nasal cavity, increase the drug concentration gradient
across the epithelium. This system is useful in intranasal drug delivery
are alginate, cellulose and alginate or its derivatives [41].
Dendrimers
Dendrimers are repetitively branched molecules, and
they exhibit improved physicochemical properties compared with typical
macromolecules. In general, Dendrimers are highly monodisperse
nanoparticles, and the size and surface functionality of the final
formulations are precisely controllable. Dendrimers are carrying a large
amount of drugs, and the PEG modified dendrimer shows favourable
pulmonary absorption after inhalation. Thus, dendrimers have been widely
used for the delivery of therapeutics for chronic lung diseases, and
anticancer agents, antibiotics, and steroids have been reported to be
delivered to the lungs by dendrimers.
Latest Development
Aradigm was developed AERx pulmonary technology,
which help in transferred insulin and morphine into the pulmonary route.
On the other side, Alkermes has prepared an inhalation technology,
which would enable us to deliver dry powder of small molecules, peptide
and protein drug particles to the deep lungs. Nektar Therapeutic in
conjunction with Pfizer began dosing first diabetic patients for the
phase III clinical trial for inhalable insulin Exubera. They are also
developing other active pharmaceuticals molecules to be delivered by
using its proprietary delivery technology. The different problems faced
by pulmonary drug delivery systems, many peptide and protein drugs are
currently investigated for potential systemic absorption by pulmonary
system, and that includes insulin, calcitonin,
luteinizing-hormone-releasing hormone analogs, granulocyte
colony-stimulating factor, and human growth hormone.
Applications of pulmonary drug delivery in Asthma and COPD
Asthma is a chronic lung disease that is categorized
under inflammation and narrowing of airways. Asthma causes periods of
wheezing, breath shortness, and coughing. Asthma affects people of all
ages, but it most often starts in childhood. COPD means chronic
obstructive pulmonary diseases, which is correlated to smoking, chronic
bronchitis and emphysema. Today's inhaled drug delivery market is
conquered by the three main classes of drug such as anticholinergic,
bronchodilators and corticosteroids. All these three classes of drugs
are only given by pulmonary route. Levosalbutamol inhalers are present
in the market to treat asthma. Titropium inhalers are present in market
to teat COPD [1,50,51].
Recent role in Pulmonary Delivery in Patients on Ventilators
Nowadays to improve inhalation coordination of
patient devices are mostly used like Baby mask. This mask is attached to
spacer for small tidal volumes and low inspiratory flow rates infant
and young Childers. We can easily give medication to child up to 2 years
by using baby masks this is recent advancements in applications of
pulmonary drug delivery.
New use of Pulmonary Delivery in Diabetes
Diabetes is a syndrome of disordered metabolism and
hyperglycaemia resulting from an insufficiency of insulin secretion or
resistance which cause the blindness, kidney disorder, nerve damage,
heart attack and other health problems. The most common form of this
therapy is twice-daily subcutaneous injections of insulin. This type of
treatment may cause pain and as a result encourages noncompliance by up
to half of the diabetics. Various companies are working on insulin
inhalers than any other insulin delivery option. Insulin inhalers would
work as asthma inhalers. The products fall into two main groups the dry
powder formulations and solution. E.g. Novel pMDI formulations for
pulmonary delivery of proteins [1,52-57].
Application of Pulmonary Delivery in Migraine
Drug Ergotamine is choice for migraine. This drug used successfully to treat migraine headache via metered dose inhaler (MDI) [58].
Application of Pulmonary Delivery in Angina Pectoris
Angina pectoris is not a disease itself it is
symptoms of myocardial ischemia and it is arises as a result of
imbalance between oxygen supply and demand of myocardium. Nitroglycerine
is a drug of choice for angina pectoris, and is given through
sublingual route. Isosorbide aerosol has also been reported useful in
hypertensive emergency [1,58].
Role of Pulmonary Delivery in Vaccines
While there was moderate interest in aerosol
vaccination 1520 years ago, progress toward application has rarely seen.
About 100vaccinesare approved in the U. S. About half of these prevent
respiratory diseases, yet all are currently injected. Recently inhaled
measles vaccine given by nebulizer [55].
Use of Pulmonary Drug Delivery System Intransplantations
Inhalation route play important role in
transplantation. Acute and chronic rejections are major problems
compromising transplant and patient survival. Aerosolized cyclosporine
is useful for reducing the risk of acute rejection [56-58].
Applications of Pulmonary Delivery in Pulmonary Arterial Hypertension
In 2004, the FDA approved Ventavis (iloprost), an
inhaled treatment for pulmonary arterial hypertension, made by CoTherix
(South San Francisco, CA, USA). In pulmonary arterial hypertension,
severe restriction of blood vessels results in early death. Iloprost
naturally dilates blood vessels [50, 59].
Application of Pulmonary Drug Delivery in Cancer Chemotherapy
Lung cancer is the leading cause of cancer death and
inhaled chemotherapy is approach for the treatment of lung cancer. A
multicentre phase I clinical trial is evaluating doxorubicin HCl
inhalation solution in lung cancer patients. As many as 4lakh lung
cancer patients a study is going on aerosolized paclitaxel solution to
mice with lung tumours. The treatment significantly reduced lung tumours
and prolonged survival. Aerosol drug delivery of the anticancer agents
like difluoromethylornithine and 5- fluorouracil reduced lung tumours in
mice 50 % and 60 %, respectively. Interleukin-2 stimulates immune
function in cancer patients, but injections cause fever, malaise, and
locals welling [50].
Gene Therapy via Pulmonary Route
Recent research in application of pulmonary drug
delivery. It holds great potential for the treatment of various acquired
and inherited pulmonary diseases. The main aim of this therapy is
treatment of cystic fibrosis. Cationic-lipid mediated CFTR gene transfer
can significantly influence the underlying chloride defect in the lungs
of patients with CFC. There are many problems to be overcome before
clinical applications are practical. Some of these are safe, successful
transfer of sufficient genetic material to appropriate tissue, adequate
gene expression, maintenance of expression over time, and efficacy of
expression [60].
Delivery of Pentamidine by Pulmonary Route
Protozoan Pneumocystis carinii (PCP) is major cause
of Pneumonia is Patients with acquires immunodeficiency syndrome.
Aerosol pentamidine is useful in treating mild PCP and prophylaxis
against PCP [61].
Inhaled Drug Delivery for Tuberculosis Therapy
Tuberculosis (TB) is infectious disease cause by
Mycobacterium tuberculosis. One third of the population is suffered from
TB, and new infections occur at a rate of one per second. New drugs or
delivery systems that will decrease the spread of tuberculosis and slow
down or prevent the development of drug-resistant strains are seriously
required. Lung lesions containing large numbers of bacteria are poorly
vascularised. Conventional therapy given by the parenteral or oral routs
May give sub therapeutic levels of ant tuberculosis (anti-TB) drugs.
Administering drugs by the pulmonary route to the lungs allows maximum
drug concentrations in the vicinity of the selesions. Supplementing
conventional therapy with inhaled anti-TB therapy may allow therapeutic
concentrations of drug to penetrate effectively into lung lesions and
treat the resident mycobacteria [62].
Nicotine Aerosol for Smoking Cessation
Smoking is injurious to the health. From ancient
times people smokes cigarette and get addicted with smoking. The reason
for cigarette smoking is Nicotine addiction, and nicotine replacement is
appealing as a means of reducing cigarette use to ultimately achieve
cessation.
Diagnostic Application Pulmonary Drug Delivery
It is use for therapeutic purpose as well as for
diagnosis purpose. For example, inhalation of aerosols of methacholine
and histamine is responsive in asthma [63].
Current Use of Pulmonary Delivery of Opioids as Pain Therapeutics
To avoid pain associated with inject able pain killer
pulmonary opioid delivery is better alternative. Clinical data of
inhaled opioids were focused on treatment of dyspnoea.They showed that
inhalation of various opioids compounds are safe even in severely ill
patients. The advent of specialized and efficient pulmonary drug
delivery systems make easy evaluation of inhaled opioids, such as
morphine and fentanyl, for management of severe pain associated with
surgery or malignant disease [64].
Among the various drug delivery approaches, pulmonary
drug delivery is one of the oldest drug delivery systems it is widely
used due to its potential advantages. This approach is based on
principle of inhalation of medication through the lungs which then
enters the bloodstream through the alveolar epithelium. A major
determining factor of this route is optimum particle size, which
regulates the targeted delivery of drug to lungs. Various drugs which
produce GI irritation can be administered by pulmonary route with
greater efficiency. The delivery device plays a major role in the
efficiency of pulmonary delivery. The most commonly used devices for
respiratory delivery are nebulizers, metered-dose inhalers, and dry
powder inhalers; these can be made adaptable for delivery of protein and
peptide drugs. Inhalable nanocarrier systems offer numerous advantages
owing to its decreased particle size. Carriers like microparticles,
nanoparticles, liposomes etc. can be used in pulmonary delivery. DPI
provides various benefits like its ease in use, cheapness, robustness.
Problem to deliver large amount of powder (around 50mg) in one breath
and to maintain stability of powder
formulation
are major challenges with DPI. Thus understanding of challenges and
overcoming, it coupled with anatomical and biopharmaceutical basics
helps in reducing the technical gaps and hence encourages the future
advancement in formulation of new improved strategy for pulmonary drug
delivery.
To know more about Open Access International
Journal of Pulmonary & Respiratory Sciences please click on: https://juniperpublishers.com/ijoprs/index.php
To know more about Open access Journals
Publishers please click on : Juniper Publishers
To know more about juniper publishers: https://juniperpublishers.business.site/


Comments
Post a Comment